
Investigators presented up to 2 years of post-marketing data on the first-ever gene therapy.

Investigators presented up to 2 years of post-marketing data on the first-ever gene therapy.
Enrollment is ongoing in the phase 1 CEDAR trial, which plans to dose the first patient later in 2022.

The AAV9 gene therapy is currently being evaluated in a clinical trial conducted by NINDS.

New data from cohort 4 showed outer retinal restoration following treatment with OpRegen.
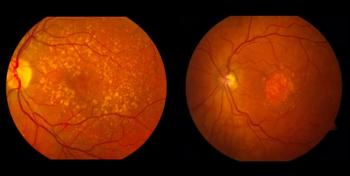
The post-hoc analysis of the OPTIC trial explored the effects of neutralizing antibodies on patients treated with ADVM-002.

The real-world analysis addresses a clear gap in data from previous and ongoing cell therapy clinical trials.

The one-time therapy is meant to address familial early-onset dementia linked to the GRN gene.

The analysis of data from ZUMA-7 demonstrated significant efficacy for axi-cel over second-line standard treatment.
The gene-edited CAR T-cell therapy is currently being investigated in a phase 2 clinical trial.
A phase 1/2 multicenter study is underway following MB-106’s IND approval.

Preliminary findings from the phase 1 clinical trial demonstrate good cell persistence and expansion in vivo.

The oral therapy is intended for high-risk hematological cancer patients.

This is the third regenerative medicine advanced therapy designation granted to AlloVir’s posoleucel therapy.

The novel regimen from BioNTech demonstrated encouraging results in patients with testicular or ovarian cancer.

The biotechnology company previously pulled out of Europe after encountering payor challenges for their gene therapies.

Review top news and interview highlights from the week ending March 25, 2022.

The draft guidance from the Center for Biologics Evaluation and Research outlines safety considerations throughout the clinical development program timeline.

The phase 1b KEYNOTE-B79 trial is investigating the allogeneic cell therapy in participants with metastatic colorectal cancer.

The FDA has approved LEXEO Therapeutics’ investigational new drug application.

The dual-mechanism of the therapy helps address both systemic and organ-specific deficits.
With numerous targets in CNS diseases and a unique partnership with gene therapy experts at UT Southwestern, Taysha is hoping to fill significant unmet needs.
The early-phase data readout follows news that the FDA recently approved its IND for its CAR-T candidate in multiple myeloma.

The first US-based patient has been dosed in Lysogene’s clinical trial of LYS-GM101, following 2 other competing trials.

The 4-day virtual conference will feature plenary presentations, workshops, and bootcamps led by cell therapy industry leaders with expertise in discovery, development, manufacturing, and more.

Interim data from a phase 1 study from Intellia Therapeutics and Regeneron Pharmaceuticals is the first to support in vivo CRISPR genome editing in humans.

The allogeneic, cellularized scaffold product helps support autologous skin tissue regeneration in patients with thermal burns requiring surgical intervention.

Locanabio CEO James Burns, PhD, discusses the firm's targeted approach to treating genetic disorders of the central nervous system and the eye.
Ross Macdonald, PhD, managing director and CEO of Cynata Therapeutics, discusses the biotech company's approach to overcoming reproducibility challenges with mesenchymal stem cells.

Maria A. Croyle, PhD, discusses the development of a novel film matrix that can safely and effectively store and transport AAV-based gene therapies.

Maria A. Croyle, PhD, discusses the manufacturing challenges facing the gene therapy space and her inspiration for developing a novel preservation method for viral vector-based therapies.