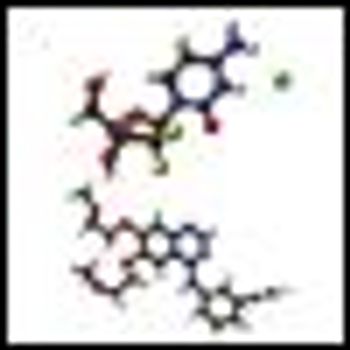
In this phase III trial, investigators assessed the clinical efficacy and safety of durvalumab with or without tremelimumab with etoposide and carboplatin or cisplatin chemotherapy followed by durvalumab with or without tremelimumab maintenance therapy compared with EP alone as first-line treatment in extensive-stage small-cell lung cancer.