Articles by Jane de Lartigue, PhD
November 24, 2020 - Until now, the field of cell-based immunotherapy has been dominated by chimeric antigen receptor (CAR) T cells, with groundbreaking FDA approvals for 3 drugs across several types of hematologic malignancies. In solid tumors, however, CAR T-cell therapies have yet to gain ground.

Investigators have turned their attention to B-cell maturation antigen, which offers an ideal target for multiple myeloma therapy because of its restricted expression pattern.

Although CD19 has proved to be an attractive and effective target for chimeric antigen receptor T-cell therapies in hematologic malignancies, a significant subset of patients treated with this groundbreaking form of immunotherapy eventually relapse.

Chimeric antigen receptor (CAR) T cells have hit prime time, with the 2 recent FDA approvals for this class of cell-based therapy.
The concept of targeting mitotic cell division to halt the progression of rapidly dividing cancer cells has long been a staple of oncology therapy, yet chemotherapy agents that are the prime examples of this approach are nonselective in their action and can kill normal and malignant cells alike.
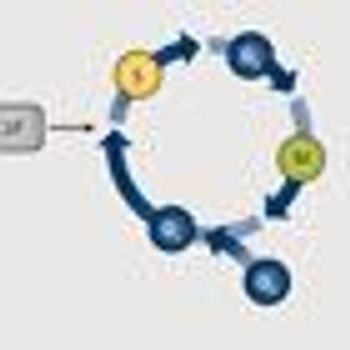
As gatekeepers of the cell cycle, the cyclin-dependent kinases are often implicated in the progression of cancer and make prime targets for therapy.

An interview with Renier J. Brentjens, MD, PhD, on new therapies for patients with acute and chronic leukemias, in particular novel immunotherapies such as chimeric antigen receptor T cells.

Five early-phase clinical trials exploring chimeric antigen receptor (CAR) T-cell therapy have been suspended temporarily in response to the deaths of 2 patients with adult B-cell acute lymphoblastic leukemia