
The chimeric antigen receptor-modified autologous T cells targeting CLDN18.2 had a manageable safety/tolerability profile and promising efficacy.

The chimeric antigen receptor-modified autologous T cells targeting CLDN18.2 had a manageable safety/tolerability profile and promising efficacy.

Regardless of bone marrow cellularity, bone marrow minimal residual disease (MRD) negativity at month 1 correlated with deep response and prolonged PFS.

Cohort A of the CARTITUDE-2 study is evaluating cilta-cel safety and efficacy in patients with multiple myeloma who received 1 to 3 prior lines of therapy.

No differences were found in overall survival and progression free survival across races.

Findings from a preclinical study, which are in contrast with the current biphasic recovery model, have implications for both HSC transplantation and gene therapy.

Investigators are evaluating the safety, tolerability and preliminary efficacy of CT-0508 in patients with solid tumors and HER2 overexpression.
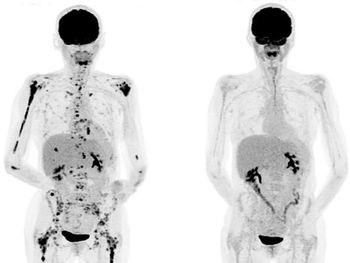
PET/CT plays a central role in evaluating response after CAR T-cell therapy.