
The autologous CAR T-cell therapy A2B530 is being investigated for solid tumors in the multicenter, first-in-human, phase 1/2 EVEREST-1 study.

The autologous CAR T-cell therapy A2B530 is being investigated for solid tumors in the multicenter, first-in-human, phase 1/2 EVEREST-1 study.

The Type II variation application for ciltacabtagene autoleucel in adult patients with relapsed and lenalidomide-refractory multiple myeloma is supported by data from the phase 3 CARTITUDE-4 trial.

CT103A, a fully human BCMA-directed CAR T-cell therapy, demonstrated deepening efficacy with an acceptable toxicity profile, according to updated data from the phase 1/2 FUMANBA-1 trial.

The approval was based on data from the phase 2 ELARA clinical trial, in which a complete response of more than 65% was observed.
Iovance plans to complete BLA submission for lifileucel by August 2022.
The investigational therapy leverages an advanced overnight nonviral gene delivery manufacturing process that may help it overcome existing treatment limitations.
Half the patients in the high-dose cohort of a phase 1a/2b trial achieved complete responses.
An interim safety review of 81 patients informed the DSMB's recommendation for the trial to continue.
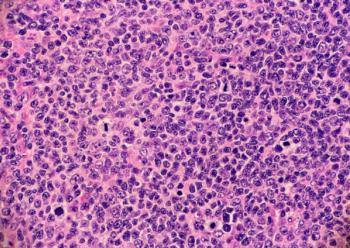
The new application is based on data from the phase 3 TRANSFORM trial.
Only 1 patient has been dosed in the trial so far, which initiated in October 2021.
Kimmtrak is now the first approved therapy for unresectable or metastatic uveal melanoma.

The approval was based on findings from the phase 2 KarMMa trial and the phase 1 Study CRB-401 trial.

The FDA has approved the prescribing label update based on data from a new safety management cohort of the phase 1/2 ZUMA-1 trial.
The T-cell therapy targets 6 tumor-associated antigens that are highly expressed in pancreatic cancer.

Positive data from a phase 1 trial were presented at the 2021 ASCO meeting.
Genprex will launch the open-label, multicenter, phase 1/2 Acclaim-2 trial in the first quarter of 2022.
The FDA also accepted a Type II Variation for the use of tisagenlecleucel in patients with R/R FL following 2 prior lines of treatment.
The FDA has pushed cilta-cel's BLA PDUFA date back by almost 4 months.
ZUMA-3 showed that a single infusion of the CAR T-cell therapy elicited a high and durable response rate in heavily pretreated patients with relapsed/refractory B-ALL.
Treatment with the CAR T-cell therapy yielded a 60% reduction in the risk of EFS events compared with SOC with a median follow-up of 2 years.

CT-0508 is currently under investigation in a phase 1 clinical trial.
The CAR T therapy tisagenlecleucel missed its EFS endpoint in the phase 3 BELINDA trial for B-cell non-Hodgkin lymphoma.
The FDA previously approved ide-cel as the first BCMA-directed CAR T-cell therapy for patients with relapsed/refractory multiple myeloma following 4 or more prior lines of therapy.

The ongoing phase 1 UNIVERSAL trial has demonstrated an overall response rate of 60% among 10 patients.
Findings from an ongoing phase 1 trial were presented at the 2021 ASGCT meeting.

The phase 3 ZUMA-7 trial met both its primary and secondary endpoints.
The recommendation comes after positive data was released from the recent phase 2 KarMMa trial.

The autologous CAR T-cell product CART-ddBCMA was found to elicit a 100% objective response rate in patients with relapsed/refractory multiple myeloma, with deep and durable responses noted in those with poor prognostic factors.

The FDA has granted a fast track designation to the CAR T-cell product AIC100 for the treatment of patients with anaplastic thyroid cancer and refractory poorly differentiated thyroid cancer.
An off-the-shelf, allogeneic CD30-CAR Epstein Barr virus–specific T-cell therapy has demonstrated favorable safety and encouraging clinical activity, even when given at lower dose levels, in patients with relapsed/refractory CD30-positive lymphoma.
Published: January 13th 2022 | Updated:
Published: June 27th 2021 | Updated:
Published: March 18th 2022 | Updated:

Published: September 5th 2018 | Updated:
Published: May 21st 2018 | Updated:
Published: August 24th 2018 | Updated: