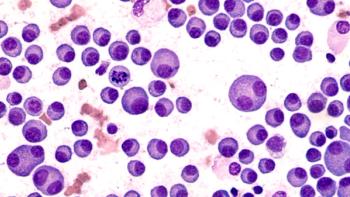
The recommended phase 2 dose of cilta-cel elicited deep and durable responses, along with a tolerable safety profile.

The recommended phase 2 dose of cilta-cel elicited deep and durable responses, along with a tolerable safety profile.
A reduction in late mortality among patients who received allogeneic blood or marrow transplantation in the last 40 years was limited to those who received treatment at a younger age.

Treatment with the CAR T-cell therapy ciltacabtagene autoleucel significantly improved outcomes for patients with relapsed or refractory multiple myeloma when compared with conventional therapies.

January 28, 2021 — Frontline pembrolizumab continued to demonstrate clinically meaningful improvements in overall survival, overall response rate, and time to progression on next-line therapy compared with platinum-based chemotherapy in patients with locally advanced or metastatic PD-L1–positive non–small cell lung cancer without sensitizing EGFR or ALK mutations.

A phase 2 study found that data from a 3-year follow up showed statistically significant improvements in overall survival for patients with high-risk locally advanced squamous cell carcinoma of the head and neck treated with an IAP antagonists with chemo-radiation therapy.

Jesus G. Berdeja, MD, of the Sarah Cannon Research Institute discussed the CARTITUDE-1 study that examined CAR-T cell therapy to treat patients with relapsed/refractory multiple myeloma presented at the 2020 ASCO Virtual Scientific Program.

Janssen Pharmaceutical Companies recently announced the FDA granted breakthrough therapy designation to JNJ-61186372, an EGFR-MET bispecific antibody, for the treatment of metastatic non-small cell lung cancer with EGFR exon 20 mutations.

Calibr received approval from the FDA to move forward with an investigational new drug to treat relapsed/refractory B-cell malignancies with a switchable CAR T-cell therapy.

Published: March 12th 2020 | Updated:

Published: June 13th 2020 | Updated:
Published: August 14th 2020 | Updated:

Published: February 7th 2020 | Updated:

Published: June 12th 2021 | Updated:

Published: January 28th 2021 | Updated: