Gene Editing Investigations Mature With heart-1 Trial for Familial Hypercholesterolemia
Verve Therapeutics plans to expand evaluations into the US after an IND clearance was delayed by a clinical hold.
Editor's note: Verve Therapeutics has since deprioritized VERVE-101 in favor of its second-generation VERVE-102 CRISPR therapy for HeFH following grade 3 adverse events in the heart-1 trial. Click here to read more.
Following the first of its kind approval of Vertex Pharmaceuticals' and CRISPR Therapeutics’ CRISPR gene-edited cell therapy exagamglogene autotemcel (exa-cel), one forerunner in the clinical trial arena is Verve Therapeutics’ VERVE-101, an investigational gene-editing therapy intended to treat heterozygous familial hypercholesterolemia (HeFH).
Jonathan Weinsaft, MD

“I think gene editing technology, whether it be CRISPR technology or other related approaches, has markedly enhanced our ability to target given pathogenic genes and perform editing, so as to correct deficits and hopefully, improve patient outcomes. We're beginning to see that in the cancer space. We haven't yet seen it as a transformative approach in the cardiovascular space. I see it as a longer-term horizon, and by that, I mean, 5 to 10 years. But, the advances over the past decade have been impressive, and I am optimistic about its potential long term,” Jonathan Weinsaft, MD, professor of medicine, Chief, Greenberg Division of Cardiology, Weill Cornell Medicine and New York-Presbyterian/Weill Cornell Medical Center, told CGTLive.
VERVE-101 is a novel, investigational in-vivo base-editing CRISPR therapy designed to permanently turn off the PCSK9 gene in the liver to reduce levels of low-density lipoprotein cholesterol in patients with HeFH, a subtype of atherosclerotic cardiovascular disease (ASCVD).
Deepak Bhatt, MD, MPH
Credit: deepakbhatt.com

"I think gene editing is going to have a tremendous impact on all of medicine, including cardiovascular medicine. And perhaps one of the first really meaningful forays into this is the heart-1 trial that examined the concept of gene editing for the disease HeFH," Deepak Bhatt, MD, MPH, director, Mount Sinai Fuster Heart Hospital and Dr. Valentin Fuster Professor of Cardiovascular Medicine, Icahn School of Medicine, Mount Sinai, told CGTLive. "This is a really important problem, a prevalent problem and one that's associated with a fair amount of early morbidity, striking people earlier on in life and also significant mortality."
Verve is evaluating the therapy in the phase 1b heart-1 clinical trial (NCT05398029), data from which were most recently presented at the American Heart Association’s (AHA) Scientific Sessions 2023, held November 10-13 in Philadelphia, Pennsylvania, by Andrew M Bellinger, MD, PhD, chief scientific officer, Verve Therapeutics.1
READ MORE: After Exa-Cel: Exploring the Next Wave of CRISPR Gene Editing Strategies
“Cardiovascular disease is cared for using the chronic care model, daily pills, intermittent injections - for decades. What Verve is looking to do is really transform or change that paradigm of care from chronic care to hopefully a one and done," Sekar Kethiresan, MD, cofounder and chief executive officer of Verve Therapeutics, told CGTLive.
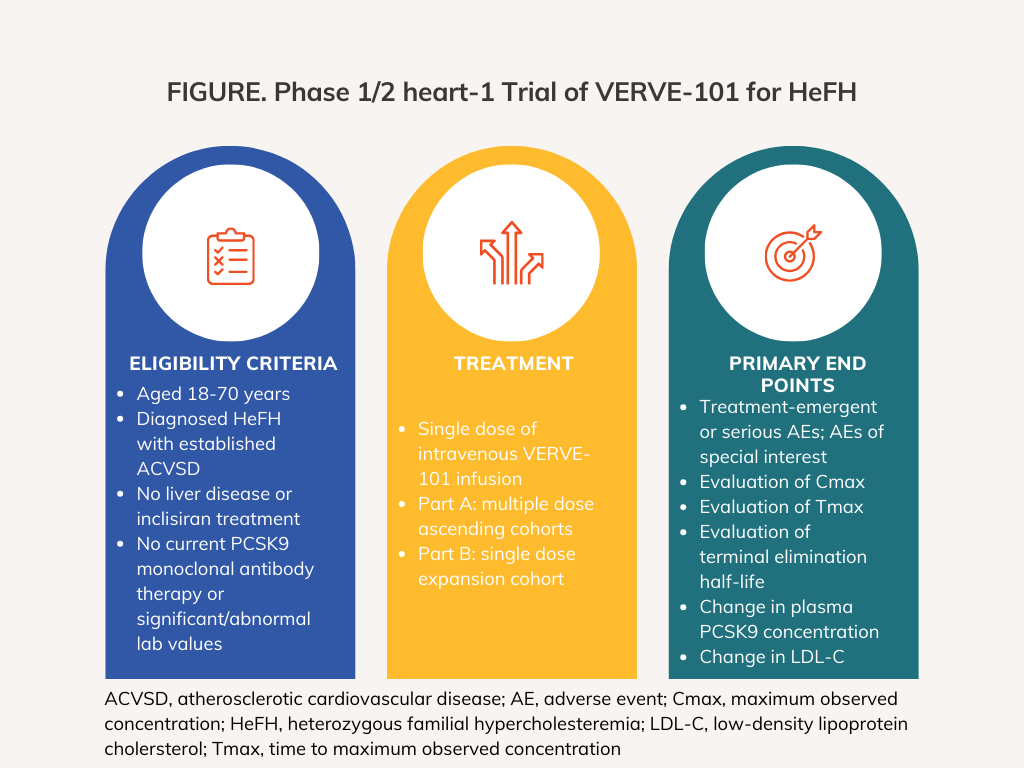
The heart-1 trial is enrolling male and female participants between 18 and 75 years of age with a diagnosis of HeFH and established ASCVD. Participants with a history of or active chronic liver disease, current treatment with PCSK9 monoclonal antibody therapy, current or past treatment with inclisiran, or clinically significant or abnormal laboratory values as defined by the protocol will be excluded from the study.
The enrolled participants, from the United Kingdom and New Zealand, had a mean age of 54 years (range, 29-69) and most were male (n = 8). They had a mean LDLC of 193 mg/dL (range, 107-373); 9 had prior coronary revascularization; 4 had more than 1 prior myocardial infarction; and 1 had prior cardiac arrest. Eight were on statin therapy and 2 had prior PCSK9-targeted therapy.1
Ten participants were treated across 4 dose cohorts - 0.1 mg/kg (n = 3), 0.3 mg/kg (n = 3), 0.45 mg/kg (n = 3), and 0.6 mg/kg (n = 1) - as of October 16, 2023.1 Enrolled patients were male and female with uncontrolled hypercholesteremia on the maximally-tolerated oral lipid lowering therapy. Participants received dexamethasone and antihistamine predications before receiving a single, peripheral intravenous infusion of VERVE-101. The trial is primarily assessing safety and tolerability as well as assessing pharmacokinetics of the therapy as well as blood PCSK9 and LDL-C change from baseline up to 1 year.
VERVE-101 decreased blood PCSK9 percentage from baseline in 2 of 3 participants in cohort 1 (+2; -9; -11) and all evaluable participants in cohorts 2 (-7; -24; -40), 3 (-59; -84) and 4 (47) from baseline at last available follow-up. Similarly, blood LDL-C decreased from baseline in 1 of 3 participants in cohort 1 (+8; +3; -3) and all evaluable participants in cohorts 2 (-9; -9; -10), 3 (-39; -48), and 4 (-55). While durability data are limited, the 55% reduction in cohort 4 has been sustained for 180 days after treatment.1
"[There is] the potential now, in the future, if this line of research continues to be promising, of editing the genome of an individual to reduce their LDL cholesterol if they've got HeFH," Bhatt said.
In terms of safety, there were 2 treatment related grade 3 or higher (serious) adverse events (AEs), which were 1 case each of cardiovascular events and increased liver transaminases. Alanine aminotransferase elevations were transient and reversible and were below the upper limit of normal. In addition, there were 2 unrelated serious cardiovascular events in 2 participant: a fatal cardiac arrest about 5 weeks after infusion that was determined to be related to ASCVD and not VERVE-101, and a myocardial infarction the day after infusion that was potentially related to therapy and then non-sustained ventricular tachycardia over 4 weeks after infusion determined to be unrelated to therapy. Common mild AEs included infusion-related reactions and upper respiratory infections or COVID-19.1
The independent data and safety monitoring board (DSMB) reviewed the safety events and recommended the trial and dosing continue. The trial is continuing to dose patients in cohorts 3 and 4, with a dose expansion cohort planned for 2024. Verve also plans to initiate a randomized and placebo-controlled phase 2 trial in 2025.
Investigations into VERVE-101 are set to be expanded to the US after the FDA cleared the investigational new drug (IND) application in October 2023, after a delay due to a clinical hold placed in November 2022 despite positive DSMB recommendations.3,4
"I think the advances in gene editing in other fields other than cardiovascular medicine, per se, will help pave the road for gene editing for cardiovascular indications... It helps the cause that there are therapies that are getting approved and other disease states," Bhatt said, referencing other advances in gene editing therapies, most prominently the approval of the first gene editing therapy exagamglogene autotemcel (exa-cel), marketed under the name Casgevy, for the treatment of severe sickle cell disease (SCD) in patients aged 12 years and older with recurrent vaso-occlusive crises and later transfusion-dependent beta thalassemia (TDT).5,6
REFERENCES
1. Vafai SB, Gladding PA, Scott R, et al. Safety and pharmacodynamic effects of VERVE-101 an investigational DNA Base editing medicine designed to durably inactivate the PCSK9 gene and lower LDL cholesterol – interim results of the phase 1b heart-1 trial. Presented at: AHA Scientific Sessions 2023; November 10-13; Philadelphia, Pennsylvania.
2. A single infusion of a gene-editing medicine may control inherited high LDL cholesterol. News release. AHA.https://newsroom.heart.org/news/a-single-infusion-of-a-gene-editing-medicine-may-control-inherited-high-ldl-cholesterol
3. Verve Therapeutics announces clearance of investigational new drug application by the U.S. FDA for VERVE-101 in patients with heterozygous familial hypercholesterolemia. News release. October 23, 2023. Accessed October 23, 2023. https://ir.vervetx.com/news-releases/news-release-details/verve-therapeutics-announces-clearance-investigational-new-drug
4. Verve Therapeutics provides regulatory update on VERVE-101 investigational new drug application and reports third quarter 2022 financial results. News release. November 7, 2022. Accessed October 23, 2023. https://finance.yahoo.com/news/verve-therapeutics-provides-regulatory-verve-113000494.html
5. FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease. News release. FDA. December 7, 2023. Accessed December 7, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
6. Vertex Announces US FDA Approval of CASGEVY™ (exagamglogene autotemcel) for the Treatment of Transfusion-Dependent Beta Thalassemia. News release. January 16, 2024. https://news.vrtx.com/news-releases/news-release-details/vertex-announces-us-fda-approval-casgevytm-exagamglogene
Newsletter
Stay at the forefront of cutting-edge science with CGT—your direct line to expert insights, breakthrough data, and real-time coverage of the latest advancements in cell and gene therapy.