
The new BLA includes “substantial longer-term data on multiple measures of neurologic benefit”, and is aimed at obtaining an accelerated approval for the gene therapy product.

The new BLA includes “substantial longer-term data on multiple measures of neurologic benefit”, and is aimed at obtaining an accelerated approval for the gene therapy product.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending January 30, 2026.

Taysha also disclosed that it has received written alignment from the FDA on elements of a potential BLA strategy for TSHA-102.

The therapy provided a +6-letter gain in BCVA-evaluable patients.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Steven W. Pipe, MD, a professor of pediatric hematology/oncology at the University of Michigan Health, also discussed open questions that remain for the future of the hemophilia B gene therapy.

Review top news and interview highlights from the week ending January 23, 2026.

For Glaucoma Awareness Month, CGTLive® interviewed the chair of ophthalmology at the Byers Eye Institute at Stanford University about the potential of cell and gene therapy for the condition.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending January 16, 2026.

We highlighted a few investigational cell and gene therapy candidates to keep an eye on in the first half of the new year.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The professor of pediatric hematology/oncology at the University of Michigan Health discussed what clinics need in order to start administering Hemgenix effectively.

Catch up on any of the key data updates you may have missed last month, with coverage highlights from the CGTLive® team.

The CRL indicates that the ALLELE clinical trial does not sufficiently show efficacy of the product.

Review top news and interview highlights from the week ending January 9, 2026.

We showcased several key moments from our on the ground coverage of the 67th American Society of Hematology Annual Meeting and Exposition.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on any of the key FDA news stories you may have missed last month, with coverage highlights from the CGTLive® team.

We highlighted a few cell and gene therapies that are nearing or likely nearing key FDA decisions in the first half of 2026.

Colleen Caleshu, MS, CGC, the senior director of research and real world data at Genome Medical, discussed important considerations for genetic counselors thinking about using AI tools in their practice.

The BLA is supported by 96-week data from the randomized, placebo-controlled phase 3 GlucoGene clinical trial.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, spoke about the potential of AI to affect development and use of cell and gene therapy products.

Take a look what stood out as pillars of progress and success from all of CGTLive's most popular oncology stories in 2025.

In this bonus episode of ImmunoLogic, the hosts discussed highlights from 2025, including some covered in previous episodes of the series.

Deborah Phippard, PhD, shared her thoughts with Renier Brentjens, MD, PhD, on cost reduction for cell and gene therapy products.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed their thoughts on the field's future.

In this bonus episode of ImmunoLogic, the hosts discussed immunotherapy highlights from ASH's annual meeting.

The trial is open to patients with a wide array of solid tumor indications.
Published: August 16th 2024 | Updated: August 19th 2024

Published: February 7th 2025 | Updated:

Published: September 30th 2025 | Updated:
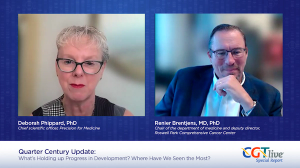
Published: October 23rd 2025 | Updated:
Published: April 24th 2023 | Updated:
Published: June 30th 2023 | Updated: