
The study portrays the intense need for daily care experienced by children with aromatic L-Amino acid decarboxylase deficiency (AADC-d) and their families.

The study portrays the intense need for daily care experienced by children with aromatic L-Amino acid decarboxylase deficiency (AADC-d) and their families.
Given that some patients may need to travel out of state to access CAR T sites of care, some may not have a clear understanding of their insurance benefits, including requirements for out-of-state or out-of-network treatment, as well as adequate assistance with the costs of medical-related travel.

Three cell and gene therapies were among the drugs included in a specialty drugs pipeline forecast at the Academy of Managed Care Pharmacy 2021 meeting.
More than 2 years after treatment, some of the patients with relapsed/refractory multiple myeloma have yet to see a relapse. An FDA decision on the therapy is expected within a month.

FDA will review the first chimeric antigen receptor (CAR) T cell immunotherapy for the treatment of multiple myeloma (MM) in adults who have not responded or have relapsed after at least 3 other therapies.

Pralsetinib, an investigational precision therapy in late-stage development for individuals with alterations in the RET gene, would be jointly sold in the United States by the 2 companies if it is approved.

The therapy is for patients with deleterious or suspected deleterious germline somatic homologous recombination repair (HRR) gene–mutated cancer or for those whose cancer has progressed after prior treatment with enzalutamide or abiraterone.

Researchers said Wednesday they created a second-generation chimeric antigen receptor (CAR) T-cell therapy that prevented relapse of lymphoma and leukemia and led to 100% long-term survival in early laboratory studies.
In a long-awaited national coverage determination decision, CMS said Wednesday that it approved chimeric antigen receptor (CAR) T-cell therapies for Medicare beneficiaries nationwide.

CMS finalized additional payments for chimeric antigen receptor (CAR) T-cell therapies and adjusted Medicare payment policies for rural and urban hospitals for fiscal year 2020 by changing the inpatient hospital wage index.
Recent work looking at the response to immune checkpoint blockade (ICB) therapy given before surgery for advanced melanoma found increased infiltration of B cells, a type of white blood cell, in patients who responded to therapy compared with those who did not.

The payment model will gather data that will be used to create a long-term model for patients not enrolled in studies or registries.
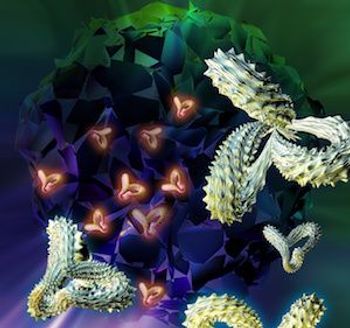
Improvements in chimeric antigen receptor (CAR) T-cell therapy, approved last year by the FDA for leukemias and lymphomas, were a focus of a session by a National Cancer Institute (NCI) researcher who spoke at the CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference taking place in New York City this week.
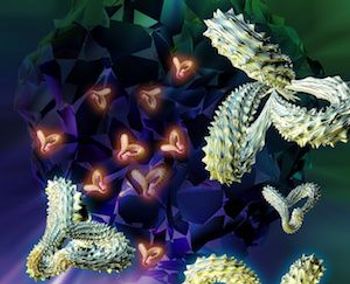
A single leukemia cell was able to reproduce and cause a deadly relapse of pediatric B-cell acute lymphoblastic leukemia (ALL) after it had bonded with the leukemia-targeting chimeric antigen receptor (CAR) lentivirus and infused back into a patient. The case of the cell that became resistant to CAR T-cell therapy was published in the journal Nature Medicine Monday.
During a meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC), panelists heard from chimeric antigen receptor (CAR) T therapy drug makers, health researchers, and policy makers, and mostly endorsed including patient-reported outcomes (PROs) in its final national coverage analysis decision, expected next year.

The first chimeric antigen receptor (CAR) T-cell therapy was approved just a year ago, changing the face of treatment for certain types of leukemias and lymphomas but carrying with it the downsides of toxicity and cost. A year later, scientists from a major cancer center said that they’ve made headway to discovering more about the T-cell signaling patterns and that understanding more about the biological pathways could help design the next generation of CAR-T treatments.

Next week, a CMS committee will hold a day-long meeting to discuss a national coverage determination (NCD) for chimeric antigen receptor (CAR) T-cell immunotherapies, and in Thursday’s New England Journal of Medicine, Peter B. Bach, MD, MAPP, reviewed several strategies open to CMS as it continues to try to determine how to pay for CAR T.

CMS announced a raft of final rulemaking Thursday for 2019, including ending the so-called 25% rule for long-term care hospitals, and approving add-on payments estimated to cost about $72 million for chimeric antigen receptor (CAR) T-cell immunotherapy.
Final updated results of the pivotal phase 2 study that led to last year’s FDA approval of the first chimeric antigen receptor (CAR) T-cell therapy were published Wednesday in the New England Journal of Medicine.
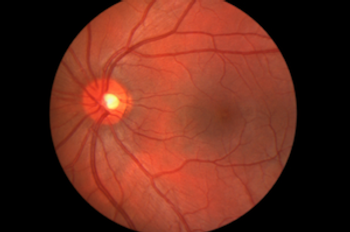
The FDA approved Spark Therapeutics Inc.’s voretigene neparvovec-rzyl (Luxturna), the first gene therapy for inherited vision loss caused by faulty gene mutations.

Published: August 3rd 2018 | Updated:
Published: March 29th 2019 | Updated:

Published: September 22nd 2020 | Updated:

Published: August 6th 2019 | Updated:
Published: October 1st 2018 | Updated:

Published: July 15th 2020 | Updated: