
Krystal Biotech's B-VEC was well-tolerated and the treated patient experienced significant improvement in visual acuity.

Krystal Biotech's B-VEC was well-tolerated and the treated patient experienced significant improvement in visual acuity.
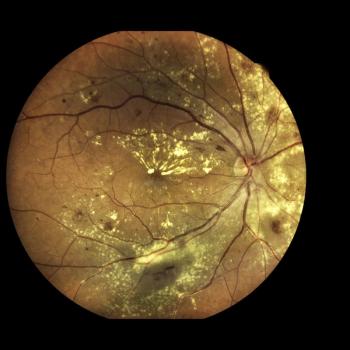
New data from imaging analyses on RG6501 were presented at the ARVO 2023 Annual Meeting.

The data, presented at ARVO's 2023 conference, also showed a favorable safety profile.

The new findings are consistent with previous research, in which ophNdi1 demonstrated benefit in AMD models.
The grant goes toward developing an innovative approach to gene-agnostically and autonomously regulate pressure.

Scientist have proposed several methods for converting stem cells into RPE, but there is still a gap in our knowledge of how cells respond to these stimuli over time.

Nanoscope Therapeutics’ Phase 2 STARLIGHT open-label trial enrolled 6 subjects with advanced vision loss due to a clinical or genetic diagnosis of Stargardt disease.

The therapy has demonstrated benefit in multiple models of dry AMD.

While focused on the retina, the researchers showed the platform works for the identification of AAVs that target tissues in various organs.
Researchers will use the model to study how lack of pigmentation affects RPE physiology and function.

Investigators did not find any clinical signs of rejection of the mismatched donor cells.

Applied Genetic Technologies is also developing AGTC-401 and AGTC-402 for the treatment of achromatopsia.
The clinical trial of 4D-150 expects to enroll around 60 participants with wet AMD.

Both the Sirius and Celeste phase 2/3 trials are evaluating QR-421a.

The ASCENT trial is kicking off while the ATMOSPHERE trial continues to screen patients with wet AMD.

ADVM-062 is designed to deliver a functional copy of the OPN1LW gene to the foveal cones of patients with BCM.

Investigators are planning a first-in-man clinical trial for photoreceptor precursor transplant in Singapore.

Researchers have developed a corneal epithelial stem cell-derived eye drop therapy for dry eye disease.
4D Molecular Therapeuitcs plans to initiate a phase 1/2 trial of 4D-150 before the end of the year.

RGX-314 is being developed to target wet AMD, diabetic retinopathy, and other chronic retinal diseases.

Investigators found that reactivating the CaMKII enzyme could protect against further vision loss.
MCO-010 is designed to deliver multi-characteristic opsin to retinal cells.
Nanoscope Therapeutics Inc. expects to advance the therapy by launching a late-stage Phase 2b trial this summer with gene therapy that delivers multi-characteristic opsin to retinal cells.

An international research team has shown that optogenetic therapy has helped to partially regain visual function in a patient with retinitis pigmentosa. This is a milestone towards a gene therapy that could restore vision.

Although the XIRIUS study did not meet its primary end point, positive trends were observed across several clinically relevant prespecified secondary end points.

ViGeneron and Biogen this week have announced a global collaboration and licensing agreement in which the partners will develop and commercialize gene therapy products based on adeno-associated virus (AAV) vectors that treat inherited eye disease.

The FDA this week granted the fourth Orphan Drug Designation for a novel gene therapy product candidate (OCU400, Ocugen Inc.) in the treatment of PDE6B gene mutation-associated retinal diseases.

Published: August 12th 2020 | Updated: