The decision was based on findings from the phase 1 TRANSCEND NHL 001 clinical trial.

The decision was based on findings from the phase 1 TRANSCEND NHL 001 clinical trial.
All patients achieved a complete response at 3 months.

Patients treated with liso-cel had a probability of continued response at 2-years of 49.5%.
Yazeed Sawalha, MD, hematologist, Ohio State University Comprehensive Cancer Center, discussed how socioeconomic factors can affect use of ASCT in mantle cell lymphoma.
Heterogeneity in the cellular and molecular features of CAR T-cell products contributes to variation in efficacy and toxicity follow treatment with axicabtagene ciloleucel.

C. Ola Landgren, MD, PhD, discussed the role of CAR T-cell therapies in multiple myeloma.
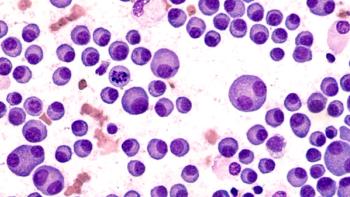
James Hoffman, MD, discussed the significance of the approval of ide-cel for patients with multiple myeloma.
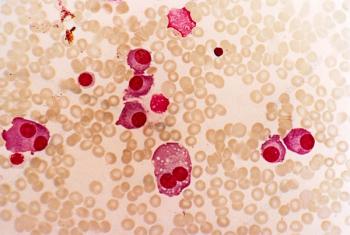
UCARTCS1A is the first allogeneic CAR T-cell product developed to target CS1 and SLAMF7, both of which are highly and consistently expressed in multiple myeloma.

Nilanjan Ghosh MD, PhD, highlights progress made with CAR T-cell therapies in B-cell lymphoma and some ongoing trials generating interest in the field.

Christopher D'Angelo, MD, discusses emerging CAR T-cell therapies in the space, as well as in follicular lymphoma, and the challenges with using a 5-drug regimen.

CD19-targeted CAR T-cell therapies have yielded durable remissions in approximately half of all patients with aggressive relapsed/refractory B-cell lymphomas.

Binod Dhakal, MD, discusses the potential impact of ide-cel, orva-cel, and cilta-cel in multiple myeloma therapy.
Responsiveness to treatment received immediately prior to CAR T-cell therapy may not be associated with post–CAR T outcomes in patients with relapsed/refractory diffuse large B-cell lymphoma who receive axicabtagene ciloleucel.

Joshua Brody, MD, discusses advances made with CAR T-cell therapy in MCL, the promise of venetoclax in B-cell malignancies, and the potential for bispecific antibody combination regimens in this disease.

The FDA has cleared an investigational new drug application for the first-of-its-kind, off-the-shelf CAR T-cell product FT819, which targets CD19-positive malignancies.

Patients with relapsed chronic lymphocytic leukemia and B-cell lymphoma who received the anti-CD19 CAR T-cell therapy FMC63-28Z experienced highly durable rates of remission, according to long-term data from a phase 1/2 study.

Published: June 21st 2021 | Updated:

Published: July 22nd 2020 | Updated:

Published: June 18th 2020 | Updated:
Published: February 9th 2021 | Updated:

Published: April 22nd 2021 | Updated:

Published: November 19th 2020 | Updated: