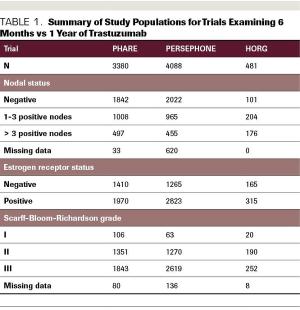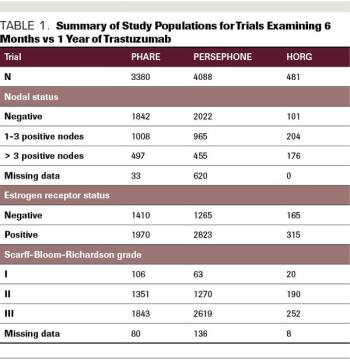
ABSTRACT Prior to the introduction of trastuzumab, the first targeted anti-HER2 agent, in 1998, patients diagnosed with HER2-positive breast cancer felt like they were being handed a death sentence. Despite treatment with aggressive chemotherapy, their tumors recurred faster, more often spread to brain and liver, and were associated with higher rates of death than HER2-negative tumors. However, in the 1980s, cancer researchers and oncologists recognized that HER2 could be targeted by a small molecule that binds to the receptor on the cell surface and blocks the signal telling the cell to divide. This small molecule was called trastuzumab, and it eventually completely changed how HER2-positive breast cancer was treated. The drug was first approved in the metastatic setting, and then the results of 2 pivotal randomized control trials demonstrated that the administration of trastuzumab in the adjuvant setting decreased the risk of breast cancer recurrence by 50%. These trials showed trastuzumab to be unequivocally effective in the adjuvant setting and the HERA trial results led to the adoption of 1 year of adjuvant trastuzumab as the standard of care. Since that time, the field of anti–HER2-targeted therapy has exploded, with the development of multiple targeted agents for use in the advanced and up-front settings. Although trastuzumab significantly improves outcomes for women diagnosed with HER2-positive breast cancer and has few adverse effects (AEs), the disadvantages are that it requires intravenous administration every 3 weeks and can be associated with cardiac AEs. It is also expensive. Given all of these factors, the question of whether a duration of trastuzumab that is shorter than 1 year may be acceptable for some patients with early-stage HER2-positive breast cancer is an important and very relevant one. Here, we will review the studies that have examined this question and evaluate their results.