
Supported by positive data from an ongoing Phase 2/3 study, bluebird bio’s Lenti-D has been granted Breakthrough Therapy designation by the US FDA for the treatment of patients with cerebral adrenoleukodystrophy.

Supported by positive data from an ongoing Phase 2/3 study, bluebird bio’s Lenti-D has been granted Breakthrough Therapy designation by the US FDA for the treatment of patients with cerebral adrenoleukodystrophy.

Agilis Biotherapeutics, Inc. has announced that the company's gene therapy for AADC deficiency results in de novo dopamine production and supports durable Improvement in major motor milestones.

New data being presented at ASGCT detail patients (1.67 to 8.42 years of age) enrolled in a study evaluating the investigational gene therapy treatment, AGIL-AADC.
Orchard Therapeutics announced that its gene therapy candidate, OTL-200, has been granted Rare Pediatric Disease designation for the treatment of metachromatic leukodystrophy.

REGENXBIO Inc. announced that the U.S. FDA has granted Fast Track designation to RGX-131, a novel, one-time investigational treatment for mucopolysaccharidosis type II (MPS II).

The U.S. FDA has cleared the Investigational New Drug application for DTX401 for the treatment of glycogen storage disease type Ia (GSDIa).

On Thursday, GlaxoSmithKline (GSK) announced that it will be transferring its portfolio of approved and investigational gene therapies to Orchard Therapeutics in exchange for a 19.9% stake in the company.

Pfizer announced that it has begun a Phase 1b clinical trial for PF-06939926, its mini-dystrophin gene therapy candidate, intended for boys with Duchenne muscular dystrophy.

A 40-year old patient at the UNC Clinical and Translational Research Center was treated with SB-913 this week.

Abeona announced that the U.S. FDA has granted rare pediatric disease designation to ABO-202, a gene therapy in development for the treatment of infantile and late infantile-onset Batten disease.
Ultragenyx Pharmaceutical, Inc. released positive long-term safety and efficacy data from the first dose cohort of the Phase 1/2 study of DTX301. The investigational AAV gene therapy, is intended for the treatment of OTC deficiency.
One month after Fibrocell submitted an Investigational New Drug application with the U.S. FDA for FCX-013, the application for the gene therapy to treat scleroderma was granted allowance.

Abeona Therapeutics, Inc. announced that the FDA has granted Orphan Drug Designation to its ABO-202 program for the treatment of infantile Batten disease.
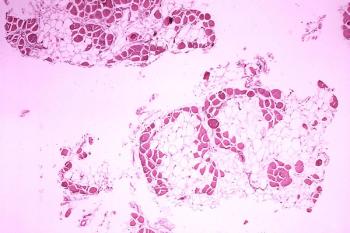
The U.S. FDA has granted Capricor Therapeutics RMAT designation for its lead investigational cell therapy for the treatment of Duchenne muscular dystrophy, CAP-1002.

Fibrocell Science announced the submission of an Investigational New Drug Application with the U.S. FDA for FCX-013, a gene therapy candidate for the treatment of moderate to severe localized scleroderma.
Rare pediatric disease designation was granted by the FDA to MeiraGTx’s A002 (ZZV2/8-hCARp.hCNGB3) for achromatopsia.

This morning, Abeona Therapeutics announced that the U.S. Food and Drug Administration granted EB-101, its gene therapy for epidermolysis bullosa, Regenerative Medicine Advanced Therapy designation.
Parent Project Muscular Dystrophy announced that the first patient with Duchenne muscular dystrophy has been dosed with microdystrophin gene therapy.

uniQure was granted Orphan Medicinal Product Designation from the European Medicines Agency for AMT-130, an investigational gene therapy for the treatment of Huntington’s disease.

In 2018, uniQure N.V. expects to advance the clinical development of AMT-130, its investigational gene therapy for the treatment of Huntington’s disease.

At the 36th Annual J.P. Morgan Healthcare Conference, uniQure announced that in 2018, the company intends to advance its gene therapy AMT-061 into a pivotal study for hemophilia B.

BioMarin Chief Executive Officer Jean-Jacques Bienaime spoke about the company’s development of valoctocogene roxaparvovec, a gene therapy in development for the treatment of Hemophilia A.
Global Blood Therapeutics, Inc. was granted Breakthrough Therapy Designation from the U.S. Food and Drug Administration for voxelotor for the treatment of sickle cell disease.

Last week, Pfizer announced a new partnership with Sangamo. As part of the agreement, the sides will team to develop a potential gene therapy to treat ALS, or Lou Gehrig’s disease.

Recent data have found that the same gene editing platform can disable the defective gene responsible for amyotrophic lateral sclerosis in mice. The therapy, which extended the lifespan of the mouse models by 25%, delayed the onset of the muscle wasting which characterizes the disease.
Published: May 3rd 2018 | Updated:

Published: January 22nd 2018 | Updated:
Published: March 6th 2018 | Updated:

Published: January 11th 2018 | Updated:

Published: April 23rd 2018 | Updated:
Published: February 1st 2018 | Updated: