
In episode 7 of ImmunoLogic, Anusha Kalbasi, MD, discussed his research on the use of IL-9 to enhance CAR-T efficacy.

In episode 7 of ImmunoLogic, Anusha Kalbasi, MD, discussed his research on the use of IL-9 to enhance CAR-T efficacy.

The research director at the Clinic for Special Children discussed how screening at-risk patients can speed up diagnosis and treatment of spinal muscular atrophy.

Catch up on any of the key FDA news stories you may have missed last month, with coverage highlights from the CGTLive® team.

Review top news and interview highlights from the week ending November 28, 2025.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, spoke about streamlining collaboration to speed up advancement of new therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
The FDA also added a new Warnings & Precaution for heightened susceptibility to serious infections caused by immunosuppression.

According to Novartis, it is the first gene replacement therapy to have been approved “for this broad population.”

Review top news and interview highlights from the week ending November 21, 2025.

The genetic counselor in the Department of Ophthalmology at the University of Pittsburgh Medical Center discussed the growing role of genetic counselors in guiding patients undergoing gene therapy.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, spoke about strategies for streamlining development of novel therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
This finding came from an analysis of 37 patients treated in the phase 1/2 LEGEND trial.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed major friction points in cell and gene therapy development and uptake in the current day.

The chair of the Division of Hematology at Mayo Clinic discussed practical considerations for the introduction of cell therapies for the treatment of hematologic malignancies.

The senior director of research and real world data at Genome Medical discussed multiple perspectives on how AI may take over some of the roles of genetic counselors in the future.

The senior director of research and real world data at Genome Medical discussed important considerations for genetic counselors thinking about using AI tools in their practice.

The senior director of research and real world data at Genome Medical discussed a session she chaired at the NSGC Annual Conference.
According to Intellia, the FDA placed clinical holds on both MAGNITUDE and MAGNITUDE-2 on October 29, 2025.

Review top news and interview highlights from the week ending November 7, 2025.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed major events in the past quarter century of cell and gene therapy research.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Naji Gehchan, MD, MBA, the chief medical and development officer of Kyverna Therapeutics, went over new data the company presented at AANEM’s 2025 meeting.
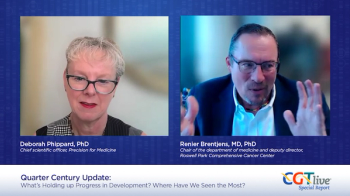
Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed how AI will affect cell and gene therapy research and practice.
uniQure pointed out that this new feedback stands in contrast to its previous communications with the FDA in several Type B meetings.

Catch up on any of the key data updates you may have missed last month, with coverage highlights from the CGTLive® team.

Catch up on any of the key FDA news stories you may have missed last month, with coverage highlights from the CGTLive® team.

Review top news and interview highlights from the week ending October 31, 2025.
Deborah Phippard, PhD, shared her thoughts with Renier Brentjens, MD, PhD, on reducing costs for cell and gene therapy products.

The chief medical and development officer of Kyverna Therapeutics went over new data the company presented at AANEM’s 2025 meeting.