
Patients who received the high dose of PBGM01 showed an increase in β-Gal activity in the CSF of 4.7 to 5.2 times baseline.

Patients who received the high dose of PBGM01 showed an increase in β-Gal activity in the CSF of 4.7 to 5.2 times baseline.

Following treatment with AT845 gene therapy, 3 of 4 patients withdrew from their previous enzyme replacement therapy.

The patient has not re-started any of his previous Gaucher-specific therapy since receiving AVR-RD-02.
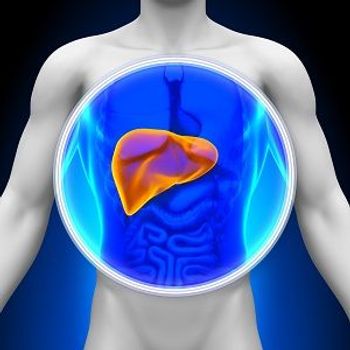
Two patients treated with AVR-RD-02 showed clinically meaningful reductions in liver size.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
By 3 months, 12 of 17 patients treated with a full cycle of EG-70 achieved a complete response.

It was found that ctDNA levels at 28 days post-treatment were significantly higher for patients who ultimately progressed by 90 days post-treatment.

Median PFS was 13.3 months in the ide-cel arm, compared to 4.4 months for the SOC arm.

Patients with large B-cell lymphoma had an ORR of 68% and a CR rate of 53%.

Among the 14 patients with relapsed/refractory mantle cell lymphoma who were treated with LV20.19 CAR in the trial, the overall response at 28 days post-treatment was 100%.

Among 43 patients who had EBV+ PTLD following allogeneic hematopoietic cell transplant or solid organ transplant, the overall response rate was 51.2%.

Neither patient treated with RP-L301 required RBC transfusions at any point post-engraftment.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
Based on 4-week follow-up data from the first patient, an independent safety evaluation committee approved the trial’s continuation.

An independent DSMB has also recommended continued enrollment in the trial’s first cohort.
No serious adverse events deemed related to RGX-314 occurred in the treated patients.

Significant reductions in pain were reported for patients who received rexlemestrocel-L+HA in comparison to patients who received a saline control.
The trial will take place at the University of Miami Health System and will be carried out in collaboration with the Diabetes Research Institute.

The updated data included a median overall survival of 26 months after treatment with brexu-cel.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

A pivotal phase 2 clinical trial for RP-A501 is expected to initiate in Q2 2023.

Patients treated with ALLO-715 achieved an ORR of 55.8%.

Cartesian Therapeutics’ Descartes-08 will be administered as 6 weekly infusions and does not require preconditioning chemotherapy.

Decibel Therapeutics expects the trial for DB-OTO to begin in the first half of 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
The dosing of additional patients in a double-blind, placebo-controlled design will help support a BLA submission for TSHA-120.

Positive preclinical data related to SENTI-202 and SENTI-401 were presented at conferences last year.

There were no deaths or safety signals identified by the DSMB for the 3 treated patients in cohort 1.
No adverse events related to the infusions of stromal cells were reported.

Cilta-cel demonstrated a significant improvement in progression-free survival over standards of care.