
Updated data were presented at the ASH 2022 annual meeting.

Updated data were presented at the ASH 2022 annual meeting.

CAR T-cell therapies are now incorporated into National Comprehensive Cancer Network guidelines as a recommended third-line strategy in this setting, with the most recent addition being lisocabtagene maraleucel.

February 15, 2021 - Betibeglogene autotemcel, a one-time gene therapy, enabled durable transfusion independence in most patients with transfusion-dependent β-thalassemia who were treated across 4 clinical studies.
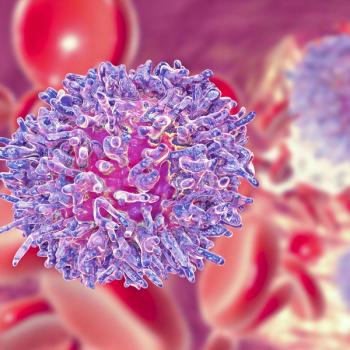
Patients with refractory large B-cell lymphoma have less to gain from axicabtagene ciloleucel if they have never achieved a complete response to any line of prior therapy.

December 18, 2020 - Sequential immunotherapy with rituximab-based therapy and the addition of lenalidomide proved effective among patients with TP53 wild-type mantle cell lymphoma, even among those with a Ki-67 level of at least 30% and/or blastoid morphology.

Vecabrutinib, a reversible, noncovalent Bruton’s tyrosine kinase inhibitor, exhibited evidence of clinical activity in adults with B-cell malignancies without producing any grade ≥3 treatment-related adverse events.

The CD19-directed CAR T-cell therapy lisocabtagene maraleucel showed promising clinical activity and manageable toxicity in heavily pretreated patients with high-risk chronic lymphocytic leukemia or small lymphocytic lymphoma, all of whom had progressed on ibrutinib.

Steroid use while cytokine release syndrome and neurologic toxicities are at grade 1, instead of waiting until grade 3, reduces the rate of CAR T-cell treatment–related CRS and neurologic events.

CD19-directed CAR T-cell therapy induced a high rate of rapid and durable complete responses in patients with aggressive relapsed/refractory large B-cell lymphoma.

Durvalumab added to etoposide and platinum-based chemotherapy as a first-line treatment for patients with extensive-stage small cell lung cancer delays development of new lesions and improves patient-reported outcomes compared with etoposide and platinum-based therapy alone.

Although CAR T-cell therapy has not yet proved effective against solid tumors, a novel CAR that targets mesothelin proteins expressed in pancreatic cancer is showing early signs of activity.
The frontline induction treatment of bendamustine and rituximab has improved event-free surviva compared with R-CHOP therapy in patients with mantle cell lymphoma.

Chimeric antigen receptor T cells targeting the tyrosine kinase receptor ROR1 can be transferred into patients safely and the cells expand in vivo.

Maintenance therapy with elotuzumab (Empliciti) and lenalidomide (Revlimid) after autologous stem cell transplant improves the quality of response achieved with induction therapy in patients with multiple myeloma.

Enzalutamide (Xtandi) added to exemestane improved progression-free survival in patients with hormone receptor-positive advanced breast cancer and no prior endocrine therapy who were positive for a gene signature-based biomarker indicating androgen receptor-signaling.

Defined composition CAR T cells directed against CD19 have potent anti-tumor activity in B cell malignancies, including acute lymphocytic leukemia.

Pembrolizumab (Keytruda) reduced the risk of death compared with standard of care therapy in patients with relapsed/metastatic head and neck squamous cell carcinoma, but the difference fell just shy of statistical significance.

Proper care coordination and patient education are essential to the success of delivering chimeric antigen receptor T-cell therapy, particularly in preparing patients for potential adverse events.

Alectinib improved progression-free survival by 66% compared with the current standard crizotinib as initial inhibitor therapy in patients with advanced or recurrent ALK-positive non–small cell lung cancer.

Anti–PD-1 therapy in the first-line induced responses in more than half of patients with advanced Merkel cell carcinoma, with encouraging durability to the responses.

Adding saracatinib to vascular endothelial growth factor (VEGF)-targeted therapy did not improve response rates or overall survival while adding to toxicity in VEGF-resistant metastatic renal cell carcinoma

Axitinib did not demonstrate superiority over sorafenib as first-line therapy for patients with metastatic renal cell carcinoma, on the endpoint of progression-free survival.

Tivozanib did not show a significant difference in overall survival when compared with sorafenib in patients with renal cell carcinoma who received up to one prior line of therapy excluding targeted agents.

Combination targeted therapy did not significantly extend progression-free survival compared with single-agent bevacizumab in patients with advanced renal cell carcinoma.

Published: February 16th 2013 | Updated:

Published: October 3rd 2019 | Updated:

Published: December 9th 2019 | Updated:
Published: December 20th 2018 | Updated:

Published: February 16th 2013 | Updated:

Published: April 19th 2016 | Updated: