
The Senior Director and Head of Technology Development at Epic Bio discussed potential applications of the new technology.

The Senior Director and Head of Technology Development at Epic Bio discussed potential applications of the new technology.

The medical director of clinical development at AskBio discussed the progress of an early clinical trial of AB-1002.

Erika Fullwood Augustine, MD, MS, the associate chief science officer of the Kennedy Krieger Institute discussed an aspect of clinical trial design highly relevant to gene therapy development for rare diseases.

The pediatric neurologist at Nemours Children’s Health discussed next steps in the field to fully enable the benefits of gene therapy.

The cofounder and chief scientific officer at Earli discussed the company’s unique approach to cancer diagnosis.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington discussed the role the ASGCT plays in the field.

Sebastien Lefebvre, MSc, the vice president of R&D at Verdot, discussed the company's unique platform.

The chief product officer at Mission Bio discussed the company’s Genome Editing Solution.

Current handling guidelines do not align between the laboratory and clinical settings.

The chief product officer at Mission Bio discussed the company’s Genome Editing Solution.

The deputy director, clinical research, San Raffaele Telethon Institute for gene therapy, discussed long-term follow-up data of up to 12 years.
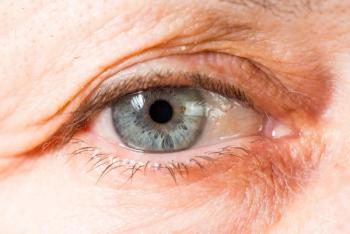
No serious adverse events or significant retinal atrophy occurred.

The CEO of Mission Bio discussed the company’s Tapestri platform for single cell sequencing.

An ASGCT poster focused on special ethical considerations of gene therapy human research and how to address them.

The investigator of neurology at Mass General Research Institute discussed research from her grad student, Lisa Nieland, presented at the ASGCT 2024 meeting.

The head of research at Mass General Brigham’s Cell and Gene Therapy Institute discussed research from the center presented at ASGCT 2024.

The cofounder and chief technology officer of Mammoth Biosciences discussed the importance of diverse approaches to gene editing to address a variety of indications.

Evan Weber, PhD, an assistant professor of pediatrics at Children's Hospital of Philadelphia, discussed his work on the role of the FOXO1 gene in T-cell persistence and exhaustion.

The chief medical officer of Forge Biologics discussed how expanded newborn screening practices may help the company’s gene therapy for Krabbe disease, FBX-101, reach more patients.

The chief scientific officer of Arbor Biotechnologies discussed the company’s preclinical candidate ABO-101.

The associate chief science officer of the Kennedy Krieger Institute discussed an aspect of clinical trial design highly relevant to gene therapy development for rare diseases.

The chief medical officer of Forge Biologics discussed updated data from the REKLAIM clinical trial evaluating FBX-101.

Abhishek Gupta, BS, the senior vice president of genetic medicines at Syneos Health, discussed common setbacks in gene therapy trials and how to overcome them.

The associate professor and associate investigator of neurology at Harvard Medical School discussed research confirming proof-of-concept with EV-AAVs.

The chief executive officer of Tenaya Therapeutics discussed the growing interest in genomic medicines in cardiology.

Oncological trials of biologics also face particularly higher rates of clinical holds.

Lawrence R. Lustig, MD, discussed promising early results from the phase 1/2 CHORD trial evaluating Decibel Therapeutics and Regenerons’ DB-OTO.

The chief executive officer of Tenaya Therapeutics discussed the company’s decision to supplement the gene transfer programs in its pipeline with gene editing programs.

The assistant professor of pediatrics at Children's Hospital of Philadelphia discussed the role of the FOXO1 gene in T-cell persistence and exhaustion.

The chief executive officer of Tenaya Therapeutics discussed the company’s research on capsids, promoters, and manufacturing improvements.