
The approved indication specifically covers adults with r/r MCL who have previously been treated with at least 2 lines of systemic therapy including BTKis.

The approved indication specifically covers adults with r/r MCL who have previously been treated with at least 2 lines of systemic therapy including BTKis.

Mark Walters, MD, a professor in residence for pediatrics at the Sickle Cell Center of Excellence at the University of California discussed gene therapy’s ongoing transformation of the treatment landscape.

The research scientist at Seattle Children’s Research Institute discussed findings from mouse research he presented at ASGCT’s 2024 conference.

In light of the IND application, Galpagos intends to bring its phase 1/2 ATALANTA-1 clinical trial (NCT06561425), which is currently ongoing in Europe, to the United States.

Jennifer L. Taylor-Cousar, MD, MSCS, a professor of internal medicine and pediatrics at National Jewish Health discussed updated data from the phase 1/2 AEROW trial of 4D-710.

Ignacio Mata, PhD, an associate professor of neurology at the Cleveland Clinic Lerner Institute, discussed the challenges that will need to be overcome to apply gene therapy to a complex neurological disorder like PD.

Among more than 100 patients with hemophilia A, no FVIII-specific responses showed associations with the assessed safety or efficacy parameters.

Review top news and interview highlights from the week ending August 23, 2024.
The study was open to patients with advanced GC/GEJ whose disease was not successfully treated with 2 or more previous lines of therapy.

Brian Kim, MBA, the chief executive officer of Mission Bio, discussed the company’s Tapestri platform for single cell sequencing.
The company expects to launch a study during the first half of next year.

Travis Drow, BS, a research scientist at Seattle Children's Research Institute, discussed mouse model research he presented at ASGCT’s 2024 Meeting.
BMS is seeking to expand liso-cel's indication in the European Union to include adult patients with r/r FL who have previously received at least 2 prior lines of systemic therapy.

Anjali Pradhan, MS, the chief product officer at Mission Bio discussed the company’s Genome Editing Solution.
The company noted that the first 3 patients with enhanced host conditioning have been dosed in the CHIRON and THETIS trials evaluating ATL001.

Vanee Pho, PhD, the chief product officer at Mission Bio, discussed the company’s Genome Editing Solution.

Following up on World Lung Cancer Day, observed annually on the first of August, CGTLive® has decided to take a closer look at this novel cell therapy.

Alexandra Collin de l’Hortet, PhD, the head of therapeutics at Epic Bio, discussed EPI-321, an investigational treatment for facioscapulohumeral muscular dystrophy.

Review top news and interview highlights from the week ending August 16, 2024.
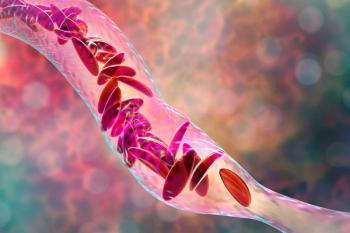
bluebird bio has reported 23 total cell collections for SCD and TDT gene therapies lovo-cel and beti-cel and Vertex Pharmaceuticals has reported approximately 20 cell collections for SCD/TDT gene therapy exa-cel.

The company reported promising early efficacy data from the Acclaim-1 and Acclaim-3 trials, but has discontinued Acclaim-2, citing difficulty enrolling patients.

David Suhy, PhD, the cofounder and chief scientific officer at Earli, discussed the company’s unique approach to cancer diagnosis.

Atsena announced initial positive data from the LIGHTHOUSE study of ATSN-201 in May 2024.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Azer-cel was originally developed by Precision BioSciences, but licensed to TG Therapeutics.
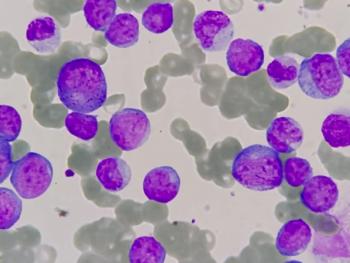
According to the company, the regulatory guidance came after a Type B meeting with the agency and pertains specifically to INB-100 for the treatment of AML.

Shankar Ramaswamy, MD, the cofounder, chairman, and CEO of Kriya Therapeutics, discussed the company’s goal of bringing gene therapy to a much broader population of patients.

A patient with MG is also the first evaluated to reach 1 year of follow-up and has a continued durable immunomodulator-free response.

BRG01 is the first cell therapy to move into a phase 2 clinical trial for relapsed/metastatic EBV-positive nasopharyngeal carcinoma in both the US and China.
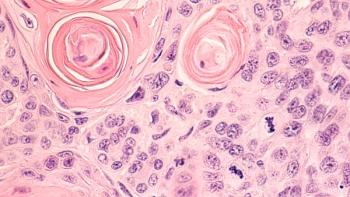
For the 5 patients in long-term follow-up who have been evaluated, the average PFS is 4.8 months (range, 2-8 months).