
The company anticipates that it will be able to submit a BLA in late 2024 or early 2025.

The company anticipates that it will be able to submit a BLA in late 2024 or early 2025.

The gene therapy is now indicated for ambulatory patients aged 4 years and older, and has been granted accelerated approval for nonambulatory patients.

The data, from the first patients dosed in each trial, also continues to show safety.
In observance of World Sickle Cell Day, CGTLive brought together a variety of expert insights on navigating the rapidly expanding landscape of care for this inherited blood disorder.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The case study was recently published in the Proceedings of the National Academy of Sciences.

The responses seen in the 9 patients included 3 complete responses (CRs), 2 very good partial responses, and 4 partial responses.

In terms of safety, SNK02 was characterized as “well-tolerated”.

Erika Fullwood Augustine, MD, MS, the associate chief science officer of the Kennedy Krieger Institute discussed an aspect of clinical trial design highly relevant to gene therapy development for rare diseases.
On the other hand, enhanced lymphodepletion increased incidence and severity of CRS and infection.

There was a 98% (2.7% SD) mean reduction in monthly HAE attack rate through the patients’ most recent assessment.

The subgroup analysis also looked at safety outcomes.
Among 10 patients who were treated with INB-100 in the trial, all 10 (100%) maintained their state of CR at 12 months or more posttreatment.
Pfizer additionally noted that the trial missed the mark on key secondary end points.

In observance of Myasthenia Gravis Awareness Month, held annually in June, we took a look back at a year of progress in bringing CAR-T to this autoimmune disease.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Ineka Whiteman, PhD, head of research and medical affairs, BDSRA Australia, discussed what has occurred in the field of Batten disease gene therapy in the past year.

Sebastien Lefebvre, MSc, the vice president of R&D at Verdot, discussed the company's unique platform.

According to recent studies and the latest insights provided by experts, early developments in gene and cell therapies show promise for patients living with Parkinson disease, but challenges remain.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
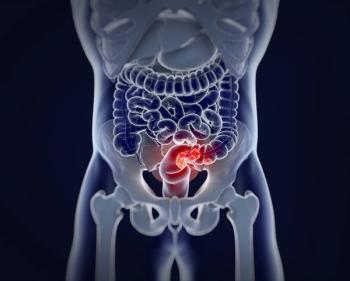
GCC19CART targets both guanylate cyclase 2C and CD19.
Imaging scans also showed disease stabilization, with no new sites of metastatic disease observed.

Ultragenyx is aiming to submit a marketing application for DTX401 next year.

The CAR T-cell therapy was also recently approved for treating follicular lymphoma, chronic lymphocytic leukemia, and small lymphocytic lymphoma.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The company also announced that it will be reporting new data at the European Hematology Association 2024 Hybrid Congress.
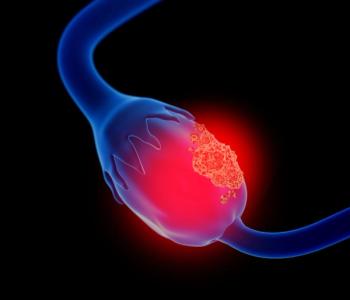
The company announced that the fifth patient was treated in its phase 1 clinical trial.

In honor of Healthy Vision Month, CGTLive® took a closer look at the clinical trial design for this novel treatment.
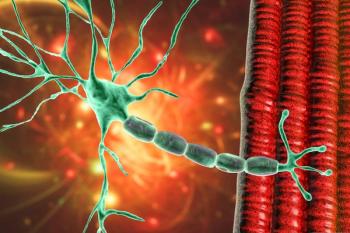
The CAR-T product is currently being evaluated in the phase 2b portion of a clinical trial (NCT04146051) for MG.

The data comes from the phase 1/2 RESET-Myositis clinical trial (NCT06154252) and the phase 1/2 RESET-SLE clinical trial (NCT06121297).