
Alexis Kuhn, PharmD, BCOP, a pediatric oncology pharmacist at Mayo Clinic, discussed the incorporation of the recently FDA-approved gene therapies for SCD and TDT into the work of pharmacists.

Alexis Kuhn, PharmD, BCOP, a pediatric oncology pharmacist at Mayo Clinic, discussed the incorporation of the recently FDA-approved gene therapies for SCD and TDT into the work of pharmacists.

The FDA has requested that black box warnings related to secondary cancer risks be added to all 6 CAR-T therapies currently on the market.

The 3 new clinical trials will take place in the United States and are intended to increase the amount of accumulated data supporting the use of Adstiladrin in NMIBC.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Aimee C. Talleur, MD, a physician at St. Jude Children’s Research Hospital, discussed obstacles to understanding late effects of CAR-T.

The specific autoimmune disease(s) that will be evaluated in the trial is as of yet undetermined, but the company hopes to initiate the trial in Q4 of 2024.

In light of the IND clearance, Tr1X anticipates initiating a phase 1 clinical trial within the second quarter of this year.

Tiffany Chen, PhD, the vice president of discovery at GentiBio, discussed the general and unique challenges of transitioning cell therapies to clinical trials in autoimmune disease.

Amar Kelkar, MD, a stem cell transplantation physician at the Dana-Farber Cancer Institute, discussed his research on the value of advanced therapeutics.

Shalini Shenoy, MD, MBBS, discussed when the choice should be made to transition from symptom management to curative therapies.

The data comes from 2 phase 1/2a clinical trials and their respective open-label extension studies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The 2-part, multicenter BCT-006-US trial will seek to enroll patients who are in the earlier stages of ALS.
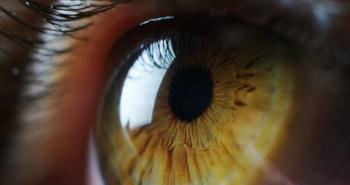
AURN001 consists of allogeneic human corneal endothelial cells referred to as “neltependocel” and Y-27632, a small molecule drug.

Atul Malhotra, MD, PhD, the head of the early neurodevelopment clinic at Monash Children's Hospital, discussed his lab’s research in the field of neonatal cell therapy.

Caribou stated that it anticipates initiating its planned phase 1 GALLOP clinical trial for CB-010 in patients with LN and ERL by the end of 2024.

Haydar Frangoul, MD, discussed advantages and disadvantages of haploidentical bone marrow transplant and the 2 new gene therapies for SCD.

Eque-cel was previously evaluated in antibody-mediated idiopathic inflammatory disorders of the nervous system in an investigator-initiated trial.

Nirav Shah, MD, an associate professor of medicine at Medical College of Wisconsin, discussed LV20.19, a bispecific CD19/CD20-targeted CAR-T being evaluated for chronic lymphocytic leukemia and Richter’s transformation.

The FDA’s decision was based on data from the phase 3 CARTITUDE-4 clinical trial (NCT04181827).

Uttam Rao, MD, MBA, a transplant physician at St. David's South Austin Medical Center of Sarah Cannon, discussed research comparing patient outcomes on different conditioning regimens for CAR-T.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Atul Malhotra, MD, PhD, the head of the early neurodevelopment clinic at Monash Children's Hospital, discussed the importance of preclinical guidelines in the context of neonatal cell therapy.

Judy Lieberman, MD, PhD, the endowed chair in cellular and molecular medicine at Boston Children’s Hospital, discussed how there is still much room for growth for RNA therapeutics despite progress so far.

In observance of Parkinson's Awareness Month, held annually in April, CGTLive® took a closer look at REGENERATE-PD, a new trial for PD gene therapy AB-1005.

Vivien Sheehan, MD, PhD, an associate professor of pediatrics at Emory University, discussed how patients should be able to choose to try the recently FDA-approved cell-based therapies for SCD when they are ready.

In light of the CTA clearance, Atamyo plans to carry out a multicenter, open-label phase 1b clinical trial (NCT05973630).

Judy Lieberman, MD, PhD, the endowed chair in cellular and molecular medicine at Boston Children’s Hospital, discussed her research on siRNA technology.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Notably, the combination therapy, referred to as SYNCAR-001 + STK-009, will be administered in the trial without the use of lymphodepletion.