
Sang Yoon Moon, PhD, postdoctoral Research Associate, Lions Eye Institute, discussed research with CRISPRa in human fibroblast cultures.

Sang Yoon Moon, PhD, postdoctoral Research Associate, Lions Eye Institute, discussed research with CRISPRa in human fibroblast cultures.

Bart P. Leroy, MD, PhD, head of the Department of Ophthalmology at the Center for Medical Genetics of Ghent University Hospital, discussed the need to reevaluate the goal posts used to determine whether gene therapies for eye indications come to market.

Bart P. Leroy, MD, PhD, head of the Department of Ophthalmology at the Center for Medical Genetics of Ghent University Hospital, discussed new real world data regarding Spark Therapeutics’ Luxturna that was presented at ARVO’s 2023 conference.

The director of clinical research at Sierra Eye Associates discussed 4D Molecular Therapeutics’ plans for 4D-150 in both wet AMD and diabetic macular edema.

Bart P. Leroy, MD, PhD, head, Department of Ophthalmology, Center for Medical Genetics, Ghent University Hospital, discussed the ongoing efforts to verify the efficacy of Spark Therapeutics’ Luxturna.

The director of clinical research at Sierra Eye Associates discussed the latest results from a clinical trial evaluating 4D Molecular Therapeutics’ gene therapy, 4D-150, that were presented at ARVO’s 2023 conference.

Arnaud Lacoste, PhD, the chief scientific officer of Aurion Biotech, discussed AURN001, a corneal endothelial cell therapy that was recently approved in Japan, and had a nonclinical data read out at ARVO 2023.

The head of the Department of Ophthalmology at the Center for Medical Genetics at Ghent University Hospital discussed the need to reevaluate requirements for regulatory approval for ophthalmology gene therapies.

Krystal Biotech's B-VEC was well-tolerated and the treated patient experienced significant improvement in visual acuity.

The head of the Department of Ophthalmology at the Center for Medical Genetics, Ghent University Hospital, discussed the main takeaways of a post-marketing study on the gene therapy.

CGTLive's sister publication spoke with Barth, the chief medical officer at Ascidian, about the company’s attempts to develop a new method of administration for gene therapy at ARVO 2023.
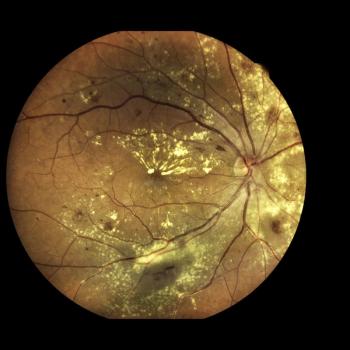
New data from imaging analyses on RG6501 were presented at the ARVO 2023 Annual Meeting.

The study found significant improvements in BCVA, in contrast to several other studies evaluating Luxturna.

The data, presented at ARVO's 2023 conference, also showed a favorable safety profile.
The chief scientific officer of Aurion Biotech discussed the properties of the cell therapy for corneal dystrophies, which was recently approved in Japan.


The new data builds upon positive long-term follow-up data from clinical trials.

The head of the Department of Ophthalmology at the Center for Medical Genetics, Ghent University Hospital, discussed the results of a post-marketing study presented at ARVO’s 2023 conference.

The safety profile of lenadogene nolparvovec at 5-years post treatment was similar to 2-year data.

No serious TEAEs have been related to ATSN-101 with data up to 6 months after treatment.
The chief scientific officer of Aurion Biotech discussed the investigational cell therapy AURN001.

STZ eyetrial’s treatment rAAV8.hPDE6A was otherwise deemed well-tolerated.

The commercially available gene therapy, developed by Spark Therapeutics, was evaluated in multiple studies presented at ARVO’s 2023 conference.

The new positive data on the AAV based gene therapy builds upon real-world data presented at ARVO’s conference in 2022.

Investigators presented up to 2 years of post-marketing data on the first-ever gene therapy.

Updated data at ARVO show that AGTC-501 had sustained efficacy and safety at 18 months for patients with X-linked retinitis pigmentosa caused by RPGR mutations.

Veeral S. Sheth, MD, MBA, Director of Clinical Research at the University of Retina and Macula Associates, discusses advanced therapies for retinal diseases.

New data from cohort 4 showed outer retinal restoration following treatment with OpRegen.
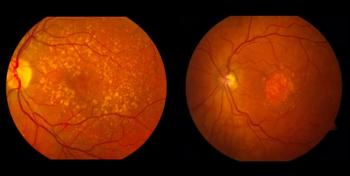
The post-hoc analysis of the OPTIC trial explored the effects of neutralizing antibodies on patients treated with ADVM-002.

The single-injection investigative gene therapy may help with providing continuous expression of aflibercept.