
Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The cell therapy is the first to come out of the company’s proprietary ARC-SparX platform.

Alexander I. Spira, MD, PhD, FACP, research institute director with Virginia Cancer Specialists Research Institute, discusses the examination of RTX-240 in a phase 1 trial (NCT04372706) with solid tumors, presented recently at the 2022 AACR Annual Meeting.

The trial was set to begin patient dosing in the first half of this year.

Tim Lu, MD, PhD, CEO and co-founder of gene circuit company Senti Bio, joins CGTL to discuss his company's approach to creating "smarter" therapies, including its CAR-NK platform.

The hold was originally placed after reports of 2 serious adverse events in pediatric patients.

Marker Therapeutics is taking a multi-antigen approach to developing next-generation T-cell therapies.

Review top news and interview highlights from the week ending May 6, 2022.

Investigators presented up to 2 years of post-marketing data on the first-ever gene therapy.

Radek Špíšek, PhD, co-founder and chief executive officer of SOTIO Global, and Geoffrey Hodge, chief executive officer of SOTIO BioTech US, join CGTL to discuss its 3 technology platforms and how they are leveraging them to attack the solid tumor micro-environment.

Updated data at ARVO show that AGTC-501 had sustained efficacy and safety at 18 months for patients with X-linked retinitis pigmentosa caused by RPGR mutations.

Veeral S. Sheth, MD, MBA, Director of Clinical Research at the University of Retina and Macula Associates, discusses advanced therapies for retinal diseases.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Although the agency has cleared the trial to restart, Pfizer will extend the voluntary hold on dosing new patients while the company talks through conditions of the restart.
Enrollment is ongoing in the phase 1 CEDAR trial, which plans to dose the first patient later in 2022.

The AAV9 gene therapy is currently being evaluated in a clinical trial conducted by NINDS.

New data from cohort 4 showed outer retinal restoration following treatment with OpRegen.
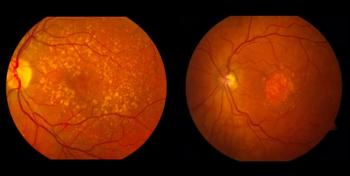
The post-hoc analysis of the OPTIC trial explored the effects of neutralizing antibodies on patients treated with ADVM-002.
The company reports positive data from first 2 patients dosed, but the agency determined there is insufficient information to support dose escalation.

There was a continued trend toward improvement of overall survival with omidubicel at 73% compared with UCBT at 60%.

Rami Elghandour, chairman and CEO of Arcellx, shares updates on the phase 1 trial of CART-ddBCMA for the treatment of subjects with relapsed and refractory multiple myeloma.

Review top news and interview highlights from the week ending April 29, 2022.

The real-world analysis addresses a clear gap in data from previous and ongoing cell therapy clinical trials.
Several CAR T-cell therapies have proved to be efficacious in hematological malignancies; however, developing a safe and effective CAR T-cell approach for solid tumors has remained a challenge.

The CEO and managing director of Chimeric Therapeutics hopes to contribute to the narrative with a handful of offerings in development across 2 platforms: CORE-NK, which uses allogeneic natural killer cells, and T-cell derived autologous therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The CIFFREO trial is underway in 11 countries but was halted in December 2021 amid safety concerns, including the death of a participant in a phase 1b study in the non-ambulatory cohort.

SQZ-PBMC-HPV receives FDA Fast Track Designation for patients with advanced or metastatic HPV16+ solid tumors, following promising early results.

Lexeo recently announced FDA clearance of its investigational new drug application for LX2006 for Friedreich's ataxia cardiomyopathy.

The one-time therapy is meant to address familial early-onset dementia linked to the GRN gene.