
A NEWDIGS analysis compared clinical trial success rates from 1988 to 2020.

A NEWDIGS analysis compared clinical trial success rates from 1988 to 2020.

Fiona Freeman, PhD, assistant professor, University College Dublin, discussed her research into microRNA-29b in suppressing tumor growth and promoting bone remodeling in mice models.

The AFFINITY trial is set to dose patients at dose level 2 by the end of 2023.

The updated data are from the first 2 patients treated in the phase 1/2 GALILEO-1 trial of FLT201.
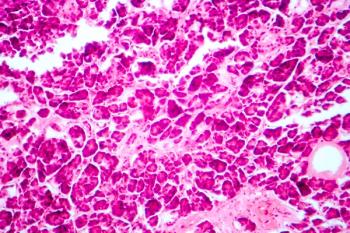
All patients showed improved glycemic control and a reduction in insulin independence.

The partnership between ASTCT, CIBMTR, and NMDP is a 2-day forum held October 2-3.
The therapy has a PDUFA date of March 31, 2024.

The new data are from the phase 1/2 MYCHELANGELO trial of OTX-2002, an mRNA OEC therapy, in patients with hepatocellular carcinoma and other MYC-expressing tumors.

Unforeseen complications may arise when treating older patients with gene therapy.

Around half of patients with R/R large B-cell lymphoma are considered ineligible for autologous stem cell transplant and are thus ineligible for the approved indication of Yescarta.

Abeona has submitted a BLA for its EB-101 cell therapy.

The phase 1/2 trial is opening its second phase of recruitment after being well-tolerated in the first 4 patients and demonstrating some vision improvements.

NFS-05 is an ophthalmic injection that uses an AAV vector to deliver the OPA1 gene to retinal ganglion cells.
The FDA issued a CRL for the therapy, to be marketed as Ryoncil, in August 2023.

Cells from patients with ALL treated in the CARPALL study were analyzed and sequenced.

Opus Genetics plans to add a pediatric cohort to the phase 1/2 trial once safety is established in adults.
The trial has dosed its third patient, and, safety validation pending, plans to dose a second cohort beginning at the end of 2023.
The FDA has accepted the company’s plan to address its current IND clinical hold on HEMO-CAR-T.
Patients were newly diagnosed and had acute myeloid leukemia positive for FLT3-iTD mutations.
Adstiladrin was approved in December 2022 and is now available for select patients under an Early Experience Program.

SynKIR-110 is based off of the KIR-CAR platform developed at the University of Pennsylvania.

Beam Therapeutics also expects to announce initial data from its BEACON trial in sickle cell in 2024.
Zilebesiran is being evaluated as a monotherapy in the KARDIA-1 study and as a combination therapy in KARDIA-2, which will report topline data in early 2024.

New data shows the ASO therapy’s benefit in older populations, following beneficial findings in patients previously treated with gene therapy.

The CAR T-cell therapy, marketed as Yikaida in China, has been approved for second-line treatment for patients with refractory/relapsed large B-cell lymphoma.

Phase 1/2 trials have been cleared in Germany and dosed first patients in the US.

In recent data from the phase 3 VITAL trial, EB-101 decreased pain and improved wound healing.

The company also recently entered into a collaboration with Roche and was invested in by Astellas.

Pending approval, ARO-DUX4 will be evaluated in patients with facioscapulohumeral muscular dystrophy and ARO-SOD1 will be evaluated in patients with amyotrophic lateral sclerosis.

Initial data from the combination substudy are expected in the second half of 2023.