The CEO of Avamab Pharma discussed how the field of gene therapy has shifted due to the COVID-19 pandemic.

The CEO of Avamab Pharma discussed how the field of gene therapy has shifted due to the COVID-19 pandemic.
Patients receiving omidubicel also spent less time in hospital following transplant.
The announcement follows positive results from the phase 1/2a trial of MCO-010 for RP.
Corlieve Therapeutics’ lead program, AMT-260, previously demonstrated proof-of-concept in preclinical studies of temporal lobe epilepsy.
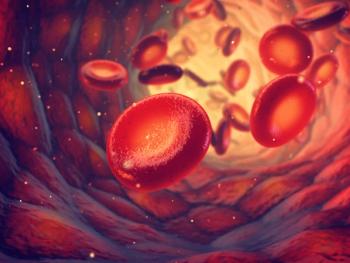
uniQure announced positive results from their phase 3 HOPE-B study of etranacogene dezaparvovec.

Jasper Therapeutics and Aruvant Sciences are studying the use of JSP191 used with ARU-1801 in patients with sickle cell disease.

The news comes after CRISPR’s favorable data readouts in its sickle cell and beta thalassemia studies.
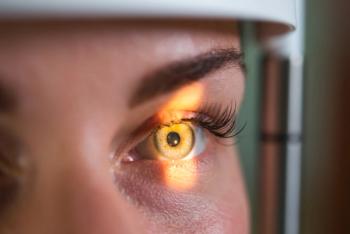
Detailed data from the phase 3 trial, including safety findings, which were in line with previous studies, will be presented at a future scientific meeting.

Bayer provided an update on trials currently underway for the treatment of PD by its subsidiaries.

The therapy utilizes LogicBio’s GeneRide technology to insert a corrective copy of the target gene via synthetic AAV vectors.
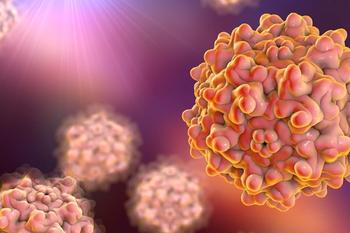
Participants in both the low- and high-dose cohorts showed improved functional outcomes at 1-year post-dose.

The partnership will allow for the development of disease-specific AAV vectors that will serve Biogen's current gene therapy pipeline.

Bijan Nejadnik, MD, chief medical officer, SanBio, also thanked neurological experts that collaborated on the phase 2 STEMTRA trial.

Masahito Kawabori, MD, PhD, associate professor, Hokkaido University, discussed results of the phase 2 STEMTRA trial.

Both therapeutic programs utilize AAD2-GDNF gene therapy targeted to brain structures vulnerable to Parkinson disease and multiple system atrophy.

There was no statistically significant difference between treatment-related adverse events in the SB623 stem cell and control groups.

Researchers from the University of Turin also found that an NEFH variant showed a protective association with ALS.

These data follow a recent announcement from Sio Gene Therapies that the first patient in the high-dose cohort has been dosed.