
The company’s pipeline is down to 1 remaining clinical-stage program that is enrolling in its first-in-human trial.

The company’s pipeline is down to 1 remaining clinical-stage program that is enrolling in its first-in-human trial.

Peter Marks, MD, PhD, director, Center for Biologics Evaluation and Research, FDA, discussed his keynote address at the 2023 MDA Conference. c
Although the TCB-202-001 trial ended prematurely due to the COVID-19 pandemic, promising results warrant a phase 2 trial.
No treatment-emergent adverse events experienced were deemed related to AstroRx, although 9 serious TEAEs did occur.

The clinical trial of HG004 for RPE65-LCA2 dosed its first patient in February 2023.
The company also recently announced updates in the MATRICS-1 clinical trial for hemorrhagic trauma.

Most patients with RP treated with MCO-010 had clinically meaningful improvements in vision.

The Study Safety Committee has recommended that the trial proceed to enroll patients in the highest-dose cohort.

The gene-edited cell therapy had previously been under a partial clinical hold restricting pediatric enrollment to the clinical trial.

Intellia Therapeutics received clearance to enroll patients from the US in the global phase 2 portion of its clinical trial earlier this month.

OCU400 is also being evaluated in other retinal degeneration indications.
The FDA will determine the acceptability of the BLA within the next 60 days.
The company's persistence in bringing NurOwn to the market amidst negative regulatory feedback has garnered an AdComm meeting.

The patient was treated at the new fifth dose level and had concomitant metapneumovirus infection.

Data from the HOPE-2-OLE were presented in a late-breaking session at the 2023 MDA Conference.

All children included in the analysis achieved swallowing, oral nutrition, and airway protection outcomes.

Individual items on RULM revealed meaningful improvements in participants from the SHINE and CHERISH studies.

New data from a single-site, first-in-human study were presented at the 2023 MDA Conference.

Data from the phase 4 RESPOND trial were presented at the 2023 MDA Conference.
Alison Betof Warner, MD, PhD, assistant attending physician, Memorial Sloan Kettering Cancer Center, discussed the solid tumor session she chaired at the 2023 Tandem Meetings.

As investigational cell therapies influx the clinical trial landscape, especially for oncologic indications, some companies are ceding the race to their competitors.

The company is back to the drawing board after discontinuing its last clinical-stage program at the end of 2022.

NR082 is being evaluated in a phase 1/2/3 clinical trial in patients with Leber hereditary optical neuropathy.

SNK01 is primarily being assessed in combination and as a monotherapy for treating solid tumors.

Competitors REGENXBIO and Capricor Therapeutics both have gene therapy candidates in clinical trials.

Intellia is also seeking an IND for phase 2 US sites of its other lead candidate, NTLA-2001 for ATTR amyloidosis.
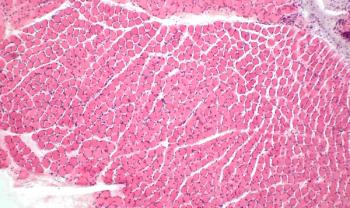
The therapy demonstrated an ability to repair injured muscle in mice models of Duchenne.

CGTLive takes a look at some upcoming FDA decisions for Rare Disease Day.

Most treated participants were within 2 SDs of normative mean in acquiring cognition, expressive language and fine motor skills.

Interim data from the phase 1/2 CAMPSIITE trial were presented at WORLDSymposium 2023.