
All evaluable patients had significant reductions in daily cornstarch intake at 1 year and at last visit during long-term follow-up.

All evaluable patients had significant reductions in daily cornstarch intake at 1 year and at last visit during long-term follow-up.

The trial compared data from external controls including a recent domagrozumab null study and a propensity score weighted comparison.

The approval was based on data from the phase 1/2 EPCORE NHL-1 clinical trial.

The most robust transduction in nonhuman primates was seen with ICV delivery of pooled PM4.AAV.mRFP and AAV2.mNG gene therapy,

Data trends suggesting stabilization of disease were seen in patients with Tay-Sachs, but more research is needed, especially for those with Sandoff disease.

Updated data from the RESTORE study on MCO-010, Nanoscope Therapeutics' investigational gene therapy, were presented at the 2023 ASGCT meeting.

ImmTOR induces immune tolerance and may reduce or eliminate Nab formation, allowing potential redosing of gene therapy.

Updated data from a phase 1 study of PCXR201, formerly known as Flexion’s FX201, were presented at the 2023 ASCGT Annual Meeting.

Committee members were mostly split, ultimately voting 8–6 in favor of the benefit-risk profile of SRP-9001 supporting accelerated approval.

The neoantigen-specific TCR therapy is the first to enter clinical trials out of a collaboration with Genentech.

Biogen and Novartis terminated collaborations with the company that first initiated in 2020.

SynKIR-110 was also granted fast track designation by the FDA in April 2023.

CK0803 is being evaluated for safety and tolerability in the REGALS study.

The draft report was released shortly after the BLA submission of exa-cel was completed and shortly before submission of lovo-cel.

Atsena anticipates initiating the Lighthouse study of ATSN-201 in mid-2023.

Updated 52-week follow-up data were presented at the EBMT meeting.


The safety profile of lenadogene nolparvovec at 5-years post treatment was similar to 2-year data.

No serious TEAEs have been related to ATSN-101 with data up to 6 months after treatment.

Overall, 6 patients were treated in a feasibility study for a disease control rate of 33.3%.

Vax-CAR-T cells significantly suppressed tumor growth in mouse models in both smaller and larger tumors.
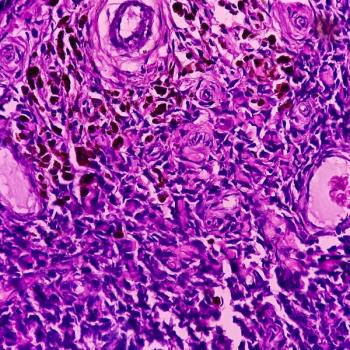
Recent data supported the TCR therapy’s pending BLA submission.

Next steps may include assessing AFM13 in combination with natural killer cells.
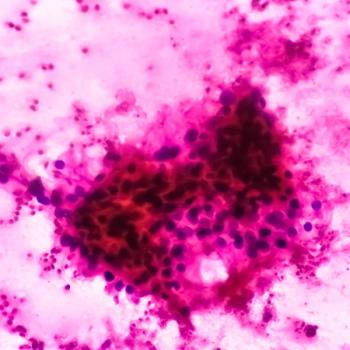
The data warrant further evaluation of ALLO-316 in patients with confirmed CD70+ tumors.

All treated eyes had stable or improved MLMT scores at 6 or 9 months of follow-up.

CGTLive takes a look at recent progress in cell therapy development targeting solid tumors, featuring expert commentary on these advances.
Matthew Gornet, MD, spine surgeon from The Orthopedic Center of St. Louis discussed data seen with IDCT cell therapy.

Vigil cell therapy previously showed some efficacy as a monotherapy in patients with ovarian cancer.

The CaMMouflage trial dosed its first participant in March 2023.

Immatics is prioritizing its TCR-T and TCER cell therapy programs and leaving behind its first-generation programs.