Forge Biologics and Solid Biosciences have also partnered to help develop a gene therapy for Duchenne muscular dystrophy.

Forge Biologics and Solid Biosciences have also partnered to help develop a gene therapy for Duchenne muscular dystrophy.
Investigators have identified the maximum tolerated dose and continue to refine the recommended phase 2 dose in the phase 1 part of the study.
Improvements in peak expiratory flow, forced expiratory volume, and forced vital capacity were observed in all evaluable treated patients in the IGNITE-DMD trial.

Patients treated with RGX-314 also had stable BCVA and central retinal thickness.

A clinical hold was previously placed after patients treated with the highest dose developed lower extremity weakness.

Improvements persisted through up to 10 years of follow-up.

The dose was previously evaluated in adult patients with no comparable inflammation.

The decision comes after 3 participants experienced serious adverse events during the phase 3 trial.

A competing RNA-based therapy for LCA10 is currently being evaluated in a phase 2/3 trial.
CAP-1002 met primary and secondary end points in the HOPE-2 trial.
Precision BioSciences is collaborating with iECURE, a new company focused on mutation-agnostic, liver disease gene therapies.
Patient-reported outcomes, 6-minute walk test, and NSAA scores all showed improvements over natural history data.
Sonny Hsiao, PhD, chief executive officer, president and cofounder, Acepodia, discussed the company’s cell therapy technologies.

The recent FDA Advisory Committee meeting follows a turbulent year for gene therapy studies.

Brian Culley, chief executive officer, Lineage Cell Therapeutics, discussed updated data from the phase 1/2 study of OpRegen.
The news comes after another of bluebird bio’s programs, eli-cel, was placed on clinical hold.

Oncternal and Celularity hope to produce ROR1-targeted NK, CAR-NK, and CAR T-cell therapies.
A phase 1 trial of a cell therapy for HIV is also currently underway.
Lineage Cell Therapeutics announced promising data from interim results of the phase 1/2a clinical trial assessing the cell therapy.
An interim analysis has met prespecified safety and efficacy checkpoints.

Following a voluntary pause, a patient experiencing a liver AE has died and the trial is now on clinical hold.
TVGN-489 is an investigational allogeneic cytotoxic CD8+ T lymphocyte therapy that may help prevent breakthrough COVID infection.
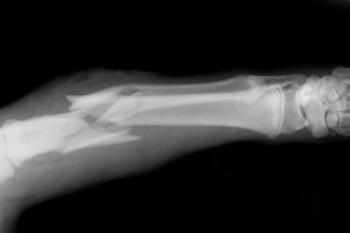
The company is currently evaluating ALLOB, its investigational cell therapy product for bone regeneration.
Paul Lammers, MD, MSc, president and chief executive officer, Triumvira Immunologics, discussed the company’s T-cell antigen coupler technology.

Decibel Therapeutics previously presented favorable preclinical data at ASGCT 2021.

Six of 7 mice treated with a new, higher dose of BMN-307 showed tumors in liver necropsy at 52 weeks post-treatment.
NeuExcell and Spark Therapeutics each add to their gene therapy pipelines with the collaborative program.
The FDA has cleared a batch of VB-111 manufactured in Modiin, Israel, following a technical review.
Both Vertex and Arbor are adding to their list of gene and cell therapy partnerships with the announcement.
The 2 companies will focus their strategic partnership on the development of gene therapies for rare diseases, as well.