Presentations at ISTH 2021 revealed attributes most important to patients when considering gene therapies and where further research is needed.

Presentations at ISTH 2021 revealed attributes most important to patients when considering gene therapies and where further research is needed.
KITE-363 is set to be investigated in a phase 1 clinical trial at the end of 2021.

Mustang Bio plans to file an IND and start a phase 1 clinical trial as soon as a lead construct is identified.

Investigators sought to understand the mechanisms of transferred T cell proliferation and expression.

Rocket Pharmaceuticals plans to quickly resume the phase 1 trial and commence dosing in their new, low-dose, pediatric cohort in Q3 2021.
A phase 1/2 study evaluating Vivet Therapeutic’s VTX-801 is set to initiate in August 2021.

The World Federation of Hemophilia’s registry will include long-term safety and efficacy data in people with hemophilia treated with gene therapies.

Bluebird Bio’s latest clinical hold follows another report of MDS in a treated patient.
Despite setbacks in DME, Adverum Biotechnologies said it will continue to develop ADVM-022 for wet AMD.
No concerning effects to liver health were observed in the phase 1/2a study.

The company announced plans to initiate the STEER study of intrathecal OAV-101 for older patients with SMA type 2.

Phase 3 studies are continuing in the enhanced Padua FIX variant AMT-061 for hemophilia B.

The designations allow for expedited drug development and review of the investigational gene therapies for CLN2 Batten disease and SOD1 ALS.

Abeona Therapeutics is seeking a path towards BLA filing based on the promising clinical data.

Bluebird Bio plans to submit its biologics license application in the US by mid 2021.
A drop in endogenous Factor VIII expression was observed from treatment to 5-year follow-up despite continued demonstration of efficacy.
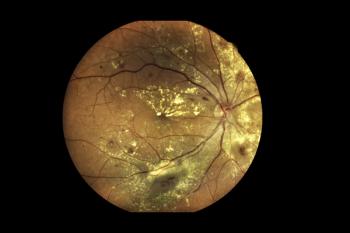
Three patients continue to show improvements in BCVA 3 years post-treatment.

The phase 1b KEYNOTE-B79 trial of CYAD-101 for mCRC is expected to initiate in Q4 2021.
Over 90% participants had an annualized bleeding rate of 0 or a lower bleed rate than baseline after week 4 after treatment.

The precision gene editing technology is designed to minimize off-target genetic alterations.

With advancements happening at unprecedented rates, these are the cell and gene therapy companies and pipelines we’re keeping a close eye on.
The clinical-stage biotech company recently announced the expansion of their phase 1 trial of CYNK-001 in patients with relapsed/refractory acute myeloid leukemia.
Janssen hopes that Vor’s stem cell therapy will allow their bispecific antibody to be better-tolerated.

The CEO of Avamab Pharma discussed how funding and research changes directions.

Study enrollment has been paused following the development of antibodies in the highest-dosed patient.

Abbvie, Biogen, and Pfizer have collaborated to launch the free resource that hosts genetic sequencing data from hundreds of thousands of participants.

The phase 3 VITAL study, which is currently enrolling, will evaluate the investigational cell therapy agent further.

Investigators found that bilaterally treated participants experienced greater improvements in BCVA than unilaterally treated participants.
The engineered natural killer cells lack the off-target effects of typical CAR T-cell therapy and similarly lack any concerning safety signals.

The FDA’s designation follows positive data presented at the 2020 ASH meeting.