
Updated data from a phase 1 study of PCXR201, formerly known as Flexion’s FX201, were presented at the 2023 ASCGT Annual Meeting.

Updated data from a phase 1 study of PCXR201, formerly known as Flexion’s FX201, were presented at the 2023 ASCGT Annual Meeting.

Vitalgen’s gene therapy was generally well-tolerated with no serious adverse events reported.

Bart P. Leroy, MD, PhD, head of the Department of Ophthalmology at the Center for Medical Genetics of Ghent University Hospital, discussed the need to reevaluate the goal posts used to determine whether gene therapies for eye indications come to market.

The cofounder and chief scientific officer of Xcell Biosciences discussed the company’s efforts to introduce new technologies for improving cell therapy development and manufacturing.

The assistant professor of stem cell transplantation at MD Anderson Cancer Center discussed positive data in ccRCC that he believes should excite the solid tumor field as a whole.

Bart P. Leroy, MD, PhD, head of the Department of Ophthalmology at the Center for Medical Genetics of Ghent University Hospital, discussed new real world data regarding Spark Therapeutics’ Luxturna that was presented at ARVO’s 2023 conference.

The director of clinical research at Sierra Eye Associates discussed 4D Molecular Therapeutics’ plans for 4D-150 in both wet AMD and diabetic macular edema.

The cofounder and chief scientific officer of Xcell Biosciences discussed the company’s manufacturing-focused approach to overcoming the challenges of the solid tumor microenvironment.

The cell therapy field applications staff scientist at ThermoFisher discussed advantages of the company’s DynaCellect system.

Bart P. Leroy, MD, PhD, head, Department of Ophthalmology, Center for Medical Genetics, Ghent University Hospital, discussed the ongoing efforts to verify the efficacy of Spark Therapeutics’ Luxturna.

The director of clinical research at Sierra Eye Associates discussed the latest results from a clinical trial evaluating 4D Molecular Therapeutics’ gene therapy, 4D-150, that were presented at ARVO’s 2023 conference.

The single-administration allogeneic cell therapy has shown positive safety—and signs of efficacy—in preliminary data from the first 2 patients dosed with the Neurona Therapeutics’ product.

Arnaud Lacoste, PhD, the chief scientific officer of Aurion Biotech, discussed AURN001, a corneal endothelial cell therapy that was recently approved in Japan, and had a nonclinical data read out at ARVO 2023.

The head of the Department of Ophthalmology at the Center for Medical Genetics at Ghent University Hospital discussed the need to reevaluate requirements for regulatory approval for ophthalmology gene therapies.

Krystal Biotech's B-VEC was well-tolerated and the treated patient experienced significant improvement in visual acuity.

The cofounder and chief scientific officer of Xcell Biosciences discussed the company’s research with a newly developed assay method that was presented at AACR’s 2023 conference.

The head of immunology at Turn Biotechnologies discussed preclinical data demonstrating proof-of-concept with the company’s epigenome reprogramming platform.

The cofounder and chief executive officer of NKILT Therapeutics discussed new preclinical data presented at WOCTC.

The head of the Department of Ophthalmology at the Center for Medical Genetics, Ghent University Hospital, discussed the main takeaways of a post-marketing study on the gene therapy.

The chief medical officer of Trisalus Life Sciences discussed the company’s PEDD system and SD-101.

Updated 52-week follow-up data were presented at the EBMT meeting.

The cofounder and chief scientific officer of Xcell Biosciences discussed the limitations of the methods currently used for modeling in preclinical cell therapy research for solid tumors.
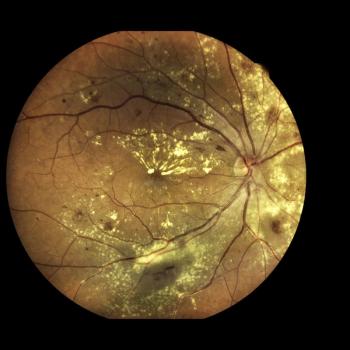
New data from imaging analyses on RG6501 were presented at the ARVO 2023 Annual Meeting.

The senior director of immunology at Arsenal Bio discussed advantages of ICTs and the company’s clinical trial in ovarian cancer.

The study found significant improvements in BCVA, in contrast to several other studies evaluating Luxturna.

The data, presented at ARVO's 2023 conference, also showed a favorable safety profile.

The vice president of business development and alliances at Prescient Therapeutics discussed the mechanism of the OmniCAR technology.
The chief scientific officer of Aurion Biotech discussed the properties of the cell therapy for corneal dystrophies, which was recently approved in Japan.


The new data builds upon positive long-term follow-up data from clinical trials.