
Long term data from 2 clinical trials and expanded access programs were presented at WORLDSymposium 2023.

Long term data from 2 clinical trials and expanded access programs were presented at WORLDSymposium 2023.

The announcement of a “strategic realignment” comes less than 2 months after the company voluntarily paused the CEDAR clinical trial due to a serious, nula-cel-related adverse event.

FBX-101 showed promising efficacy and has been well-tolerated so far in the RESKUE trial.

The gene therapy was approved in the US in the same indication in November 2022.

No adverse events related to RP-L201 have been reported to date.
Antiviral responses increased until peaking at week 24.

A third price model to be tested will limit the price of generic drugs for chronic conditions to $2 under Medicare plan D.

CT103A recently demonstrated safety in a phase 1 trial treating participants with NMOSD.

Encouraging efficacy signals have also been observed in treated participants.
The latest data set includes 22 newly evaluable patients with triple-refractory disease.

The POLARIS study adds to the growing body of evidence validating GPRC5D as a target for CAR T-cell therapy in R/R MM.

The FDA cleared Century’s IND for CNTY-101 in August 2022.

GC012F recently showed efficacy in newly-diagnosed MM in investigator-initiated trials.

The clinical hold comes a few weeks after the company announced it was stopping enrollment in the phase 1/2 clinical trial.
The phase 1 clinical trial is the second of Frontera’s trials to begin dosing in 2023.

Mesoblast is resubmitting its BLA with new CMC, survival, and mechanism of action data on the therapy and phase 3 trial.

AB-101 is also being investigated with the innate cell engager AFM13 for CD30 lymphomas.

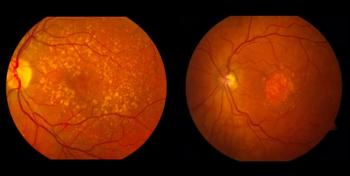
MCO-010 is in phase 2 clinical trials for treating retinitis pigmentosa and Stargardt disease.

Sana Biotechnology also plans to submit another IND for the same indications for a CD22-targeted CAR T later in 2022.
The phase 1 portion of the trial assessing CNA3103 will start enrollment in Australia in the first half of 2023; the phase 2 portion will expand to the US.
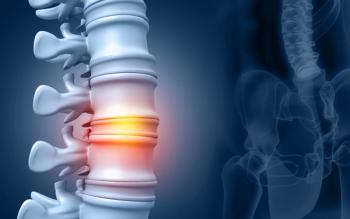
The DGX-A01 study met its primary safety and efficacy endpoints in its highest dose cohort of IDCT.

Breyanzi is currently approved for the second-line or later treatment of large B-cell lymphomas.
Iovance is on track to complete its rolling BLA submission for lifileucel in the post-anti-PD1 melanoma indication in the first quarter of 2023.

The CD19/CD20 dual-targeted CAR is currently under investigation in an investigator-initiated study in UCLA.

The FAD has granted primary IND clearance to the company’s T-Plex program and 2 initial TCR-T therapies.

NGN-401 is Neurogene’s second investigational gene therapy to enter clinical trials.

With confidence building, numerous cell and gene therapies will likely go before the FDA and other global regulatory agencies this year, in addition to key data readouts.

The FDA has lifted a clinical hold placed in the summer of 2022 due to a mild but medically significant case of peripheral sensory neuropathy.

The company is reprioritizing to focus on its clinical stage programs, which ran into a number of setbacks in 2022.
Investigators analyzed data from 3 phase 3 studies and a long-term follow-up study.