
The trial is being conducted at the University of California – San Diego under the leadership of Stephanie Cherqui, PhD.

The trial is being conducted at the University of California – San Diego under the leadership of Stephanie Cherqui, PhD.
The DMC has recommended the FREEDOM-1 trial to continue without restrictions as the patient was treated before protocols were amended to mitigate GvHD risk.

The first 6 patients dosed in the ANTLER trial had a 50% CR rate at 6 months.

KYV-101 was developed using the construct Kyverna licensed from the NIH in January 2022.

NKGen plans to initiate a phase 1 trial pd SNK02 in the first quarter of 2023.

The therapy is currently being evaluated in the phase 1/2 ARYA-1, ARYA-2, and ARYA-3 studies in adults and children.

The first patient has been dosed in a phase 2 study of the allogeneic CAR T-cell therapy GC007g.
Future data from other participants with LCA enrolled in the phase 1/2 study will be announced at a later date.
The first patient dosed with IOV-4001 has completed the safety observation period.

The PDUFA target date is set for March 31, 2023.

All 5 patients on ERT at study start have since been able to withdraw after gene therapy treatment.

Both botaretigene sparoparvovec and JNJ-81201887 were well-tolerated in treated patients, according to data from the 2022 AAO meeting.

ORR was 80% in the first cohort of phase 1b at the recommended phase 2 dose.

A retrospective study measured correlations of ICANS and NfL in patients with a history of diffuse large B-cell lymphoma.
Lyell will soon initiate a phase 1 trial of LYL845 with initial data expected in 2024.

Interim data from an ongoing study demonstrate continuing efficacy in B-NHL, CLL, and FL.

The new, 2-year data is from a roll-in cohort of 10 patients presented at the HFSA 2022 meeting.

Oncternal will soon initiate a phase 1/2 dose-escalation study of ONCT-808.

Rocket Pharma presented updated data from adult and pediatric patients at the 2022 HFSA meeting.

Solid has also pivoted from developing SGT-001 to SGT-003 for treating Duchene muscular dystrophy.
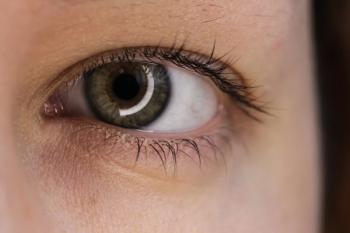
Participants in the AAVIATE trial had up to an 85% decrease in treatment burden 6 months after treatment with RGX-314.

Disease-free survival was 56% in 105 participants over 3 years.

Patients treated with the highest dose of MIC-Lx had lymphocyte reactivity against third-party cells but not stimulatory donor blood cells.

Verismo plans to initiate a phase 1, first-in-human trial of SynKIR-110 in the first quarter of 2023.

SRP-9001 has shown safety and efficacy across multiple studies compared to controls, with additional efficacy data expected in 2024 from the EMBARK trial.

A phase 1 and phase 1b study of CK0803 will soon be initiated following the FDA’s IND clearance.

CTX-130 is being investigated in phase 1 studies for relapsed/refractory T or B-cell malignancies and renal cell carcinoma.

Bluebird bio’s ZYNTEGLO was the first gene therapy to be approved in the space in August 2022.

Although efficacy and HGF expression were assessed as outcome measures, comparisons were not able to be made between placebo and Engensis groups.

Xue Zhong Liu, MD, PhD, and research partner Zheng-Yi Chen, PhD, will investigate gene editing and CRISPR/Cas9 systems to potentially treat Usher syndrome.