
Erika Fullwood Augustine, MD, MS, the associate chief science officer of the Kennedy Krieger Institute discussed an aspect of clinical trial design highly relevant to gene therapy development for rare diseases.

Erika Fullwood Augustine, MD, MS, the associate chief science officer of the Kennedy Krieger Institute discussed an aspect of clinical trial design highly relevant to gene therapy development for rare diseases.

Earlier and prophylactic interventions have enabled low rates and severity of AEs in recent years of administration.
On the other hand, enhanced lymphodepletion increased incidence and severity of CRS and infection.

The subgroup analysis also looked at safety outcomes.

The medical oncologist and clinical director of Myeloma Cellular Therapies at Dana-Farber Cancer Institute emphasized promising safety in patients with high-risk disease.

The pediatric neurologist at Nemours Children’s Health discussed next steps in the field to fully enable the benefits of gene therapy.

The lead scientist at Percheron Therapeutics discussed research on antisense oligonucleotide therapies in mouse models of DMD.

The cofounder and chief scientific officer at Earli discussed the company’s unique approach to cancer diagnosis.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington discussed the role the ASGCT plays in the field.

Sebastien Lefebvre, MSc, the vice president of R&D at Verdot, discussed the company's unique platform.
Candel also recently received orphan drug designation for CAN-3110 for treating recurrent, high-grade glioma.

The chief product officer at Mission Bio discussed the company’s Genome Editing Solution.

The professor of pediatric hematology/oncology at CS Mott Children’s Hospital discussed a sub analysis of the HOPE-B trial.

Nine responses were ongoing as of the March 2023 cutoff date.

The assistant professor at Moffit Cancer Center discussed the subgroup analysis she presented at ASCO’s 2024 Annual Meeting.
GCC19CART targets both guanylate cyclase 2C and CD19.
Of 300 screened participants, 68 have been matched by target antigen and HLA expression to a TCR-T in the ImmunoBank.

Patients with large B-cell lymphoma in the TRANSFORM trial showed improvements over 3-year period compared with standard of care.

The associate professor of medicine at University of Colorado discussed updated follow-up data from the phase 3 TRANSFORM trial presented at the 2024 ASCO meeting.

PFS jumped from under 3 months to over 14 when participants received CB-010 from a donor with at least 4 matched HLA alleles.

The McCaw Endowed Chair of Muscular Dystrophy at University of Washington shared his outlook on the trajectory of research in the field.

The CAR T-cell therapy AIC100 demonstrated promising responses and a low level of toxicity in patients with advanced thyroid cancer.

The assistant professor at Mayo Clinic School of Medicine shared her outlook and predictions on research with T-cell lymphomas.

Current handling guidelines do not align between the laboratory and clinical settings.

The chief product officer at Mission Bio discussed the company’s Genome Editing Solution.

The deputy director, clinical research, San Raffaele Telethon Institute for gene therapy, discussed long-term follow-up data of up to 12 years.
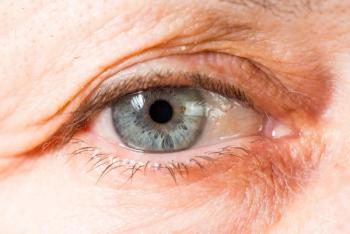
No serious adverse events or significant retinal atrophy occurred.

The CEO of Mission Bio discussed the company’s Tapestri platform for single cell sequencing.

An ASGCT poster focused on special ethical considerations of gene therapy human research and how to address them.

The investigator of neurology at Mass General Research Institute discussed research from her grad student, Lisa Nieland, presented at the ASGCT 2024 meeting.