
A novel treatment method called stem cell educator therapy appears to reverse the effects of type 1 diabetes, researchers at the University of Illinois at Chicago and colleagues in China report.

A novel treatment method called stem cell educator therapy appears to reverse the effects of type 1 diabetes, researchers at the University of Illinois at Chicago and colleagues in China report.

In this video, Dr. Neil Riordan, Founder and President of the Stem Cell Institute in Panama, discusses the scientific rationale for using adipose tissue-derived stem cells and T-regulatory cells to treat MS and rheumatoid arthritis.

Researchers in Israel have completed a clinical trial that successfully tested the use of gene therapy to restore sight to patients suffering from Leber's congenital amaurosis (LCA).

Multiple myeloma (MM) remains incurable despite the current approaches used in initial therapy, including more effective induction therapy, one or more autologous stem-cell transplants, and consolidation/maintenance strategies.

In a Cleveland Clinic video, William Rigby, MD, of Dartmouth Medical School, discusses B-cell and T-cell-directed therapy for rheumatoid arthritis.

Diffuse large B-cell non-Hodgkin lymphoma is typically a chemotherapy-sensitive malignancy, justifying dose-intense therapy with hematopoietic stem cell transplantation for patients unlikely to achieve cure with standard-dose regimens.

Stem cell-based therapy is an important potential treatment to restore vision in patients with a wide range of retina disease.
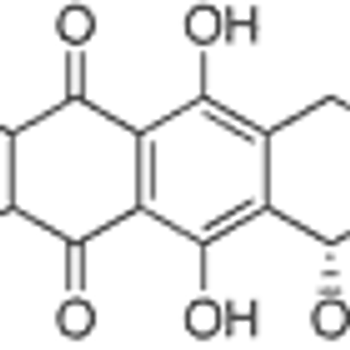
Amrubicin improved response rates, improved progression- free survival (PFS), and achieved enhanced symptom control with acceptable toxicity compared with topotecan.


The authors of "ALK-Targeted Therapy for Lung Cancer: Ready for Prime Time," in this issue of ONCOLOGY, address the newest developments in the field of targeted therapies for advanced non–small-cell lung cancer (NSCLC).
A study published online on May 10, 2011 in the British Journal of Cancer has shown evidence for a new prognostic factor in breast cancer: an increase in cancer stem cell population after primary systemic therapy. The study results indicate that putative cancer stem cells may be chemoresistant to conventional anthracycline-based chemotherapy and may have a role in disease progression following chemotherapy treatment.

Yankees' pitcher Bartolo Colon has resurrected his career with the help of a disputed therapy involving stem cell injections.

My 2002 article provided an overview of the various interleukin-2 (IL-2)–based treatment regimens that had been explored over the preceding two decades

Study shows that injection of experimental gene therapy reduces pain symptoms in patients with persistent, severe cancer pain.
The march toward personalized cancer care may have taken a step forward with the discovery of a potential biomarker

A gene therapy called NLX-P101 dramatically reduces movement impairment in Parkinson's patients.

A model example of personalized cancer therapy that has demonstrated improved patient outcomes is the use of anti-HER2 treatment. Breast cancer patients screened via molecular diagnostics and identified as having amplification of the HER2 gene generally have a poorer prognosis, but show better responses to anti-HER2 treatment.

Imatinib (Gleevec) improves the ability to proceed with allogeneic stem cell transplantation and improves 5-year overall survival (OS) when used as induction therapy in patients with Philadelphia chromosome positive (Ph ) acute lymphoblastic leukemia

Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality in the United States.

Non-small cell lung cancer (NSCLC) is diagnosed at an early stage, when it is amenable to surgical resection in approximately 20% to 25% of cases.

In the first in-human study fo human umbilical tissue-derived cells as cell-based therapy for retinal degenerative diseases, all seven patients tolerated the subretinal injection well and had no postoperative visual loss.

The dream that stem cell therapy may someday be used to protect against or restore glaucoma-related vision loss seems to be moving closer to reality.

Patients with advanced non-small-cell lung cancer achieved a significant increase in survival time when tumor treating fields (TTF) therapy was added to their chemotherapy. In a single-arm, phase II study, physicians delivered TTF therapy, using the NovoTTF-100L from Novocure, to 42 patients with stage IIIb-IV metastatic NSCLC who had failed prior treatments. Patients in the study received TTF therapy for 12 hours a day in combination with pemetrexed (Alimta) every three weeks until disease progression.

Research from Japan documenting remarkable survival rates among patients with inoperable lung cancer may only hint at the potential of proton-beam radiation therapy. The study out of the Proton Medical Research Center in Tennoudai, Japan, documented high survival rates for 55 patients suffering from stage I inoperable non-small-cell lung cancer.
Patients with incurable NSCLC are less likely to progress to second-linetherapy with the right maintenance regimen. But maintenance therapyalso means committing patients to continuous treatment without anybreaks or chances to recover from adverse events.

CAL-101 and GA101 demonstrate active results in indolent B-cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia while a secondary analysis of a pralatrexate (Folotyn) trial shows a benefit for peripheral T-cell lymphoma patients who fail second-line therapy.

The effectiveness of RT in the palliative setting is sometimes overlooked; however, RT can provide excellent palliation for patients whose disease becomes refractory to other modalities.

Despite the fact that elderly patients comprise over 50% of the non-small cell lung cancer (NSCLC) population, our knowledge regarding the efficacy and safety of chemotherapy in this group is suboptimal. The “elderly” (defined as individuals ≥70 years of age) experience physiologically normal aging of their bone marrow and kidneys, which inherently increases toxicity to therapy. Standard practice has often been to discourage the use of combination chemotherapy in these patients; however, general consensus guidelines emphasize that performance status should primarily guide the choice of treatment. Elderly patients with advanced NSCLC treated with platinum doublet therapy demonstrate similar efficacy (but increased toxicity) to their younger counterparts. Patients with metastatic disease in which a targeted and/or biological agent(s) was added to chemotherapy experienced benefits similar to those treated with standard platinum doublets, but with increased morbidity and mortality. In the future, effective testing of molecular targeted therapies will have to include elderly patients among research cohorts or risk excluding a large population of eligible patients. Overall, elderly patients with advanced NSCLC, while experiencing greater toxicity, demonstrate the same response rates and survival benefits as their younger peers.

The role of radiation therapy (RT) in lung cancer is long established; some of the earliest Radiation Therapy Oncology Group reports dealt with non-small cell lung cancer (NSCLC).[1,2] More recently, the advent of stereotactic body RT (SBRT) techniques has provided significant local control rates after focused treatment of selected small metastases and inoperable early stage lesions.[3,4] Our center has been in the forefront of examining SBRT and its role in central [5] or bilateral [6] lesions, its effect on PET imaging [7] and pulmonary function testing,[8] and subsequent frequency of brachial plexopathy,[9] chest wall toxicity,[10] or pneumonitis.[11] Still, even this highly conformal technique comes with potentially significant dose to adjacent normal tissue. This is in the context of an emerging appreciation for the pulmonary consequences of elevated mean lung dose,[12] or V5 after pneumonectomy.[13] For each lung cancer patient requiring RT, an effective mechanism to deliver dose to the tumor while minimizing dose to uninvolved lung is called for. Enter protons.

Proton radiation for cancer offers the ability to conform the high-dose region of radiation therapy to the tumor while reducing the dose of radiation to adjacent normal tissues. In lung cancer, this equates to greater sparing of uninvolved lung, heart, esophagus, and spinal cord. Sparing these normal tissues permits the delivery of higher-radiation doses to the tumor. Studies that compare the distribution of radiation doses for lung cancer show that proton radiation is superior, even when factors such as respiratory motion are considered. Clinical experience confirms the feasibility of proton radiation for early-stage non-small-cell lung cancers, and clinical trials are being conducted in locally advanced tumors: To date, evidence indicates that proton radiation should be further explored.