
In this video from the 2014 ASH Meeting, Dr. Grupp discusses data from a trial using CAR T-cell therapy in children and young adults with relapsed, treatment-resistant ALL.

In this video from the 2014 ASH Meeting, Dr. Grupp discusses data from a trial using CAR T-cell therapy in children and young adults with relapsed, treatment-resistant ALL.
Early consolidation therapy with brentuximab vedotin after autologous stem cell transplant improved progression-free survival of patients with Hodgkin lymphoma.

The anti-CD19 chimeric antigen receptor-modified T-cell therapy CTL019 demonstrated an impressive 92% complete response rate in pediatric patients with relapsed/refractory acute lymphoblastic leukemia.

The top research being presented at the 2014 American Society of Hematology Annual Meeting will focus on immunotherapies and novel agents, according to Marcel R.M. van den Brink, MD, PhD.

The chimeric antigen receptor (CAR) T cell therapy JCAR015 has received a breakthrough therapy designation from the FDA as a treatment for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).

Up until now, these treatments have not produced promising efficacy results in clinical trials. MRI could show the way.
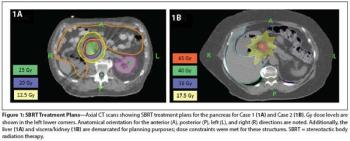
In this edition of our ongoing series, the authors present two cases involving renal cell carcinoma patients treated with SBRT for pancreatic metastases.

Ninety percent of patients with relapsed/refractory acute lymphoblastic leukemia achieved complete remission after a T-cell therapy treatment targeting CD19.

Today, Merck announced that Keytruda has been granted breakthrough therapy designation for treating patients with epidermal growth factor receptor mutation-negative and anaplastic lymphoma kinase rearrangement-negative non-small cell lung cancer (NSCLC).

The FDA has granted a breakthrough therapy designation to pembrolizumab (Keytruda), an anti-PD-1 therapy, for the treatment of patients with non-small cell lung cancer (NSCLC) who are EGFR mutation- or ALK rearrangement-negative and whose disease has progressed on or following platinum-based chemotherapy.

The FDA granted breakthrough therapy designation to pembrolizumab (Keytruda), an anti-PD-1 agent for the treatment of patients with non-small-cell lung cancer who do not have EGFR mutations or ALK rearrangements.

Stem cell therapy has gained increasing traction in various therapeutic areas, from cancer to diabetes to ocular regeneration.

Adding saracatinib to vascular endothelial growth factor (VEGF)-targeted therapy did not improve response rates or overall survival while adding to toxicity in VEGF-resistant metastatic renal cell carcinoma
The concept of targeting mitotic cell division to halt the progression of rapidly dividing cancer cells has long been a staple of oncology therapy, yet chemotherapy agents that are the prime examples of this approach are nonselective in their action and can kill normal and malignant cells alike.

A 71-year-old woman presented with back pain and was incidentally found to have a left upper pole renal mass. She underwent left open partial nephrectomy; the pathology results revealed a 2.2-cm clear-cell renal cell carcinoma (RCC) with negative margins and a Fuhrman nuclear grade of 2.

Current novel therapeutics for the prevention and treatment of bone loss in patients with inflammatory joint disease target cytokines and other inflammatory mediators. Mesenchymal stem cell therapy is a compelling new treatment currently being studied in clinical trials.
A pair of medical experts – one from industry and one from academia – have teamed up to call for a new payment model for expensive gene therapies.

Bayer Healthcare and Dimension Therapeutics recently announced a partnership to advance innovative treatment options for patients with hemophilia A.

Steven A. Rosenberg, MD, PhD, discusses the implications of responses to the CAR T-cell therapy KTE-C19, particularly among patients with chemotherapy-refractory diffuse large B-cell lymphomas.
Chimeric antigen receptor T-cell therapy is an immunotherapy in which the patient's own T cells are isolated in the laboratory, redirected with a synthetic receptor to recognize a particular antigen or protein, and reinfused into the patient.

Immunotherapy is rapidly emerging as a very attractive and novel therapeutic approach for cancer, including for glioblastoma multiforme (GBM), the most common primary malignant brain tumor in adults.

Treatment with KTE-C19, a CD19-targeted CAR-modified T cell therapy, demonstrated an objective response rate of 92% in patients with advanced B-cell malignancies.

The treatment of inoperable stage III non–small-cell lung cancer (NSCLC) remains a challenge due to high rates of distant metastasis, local recurrence, and toxicity associated with definitive therapy.
Results of a head-to-head comparison of first-line treatment of metastatic renal cell carcinoma with the mTOR inhibitor everolimus or VEGF inhibitor sunitinib showed that everolimus did not meet noninferiority requirements as a first-line therapy.
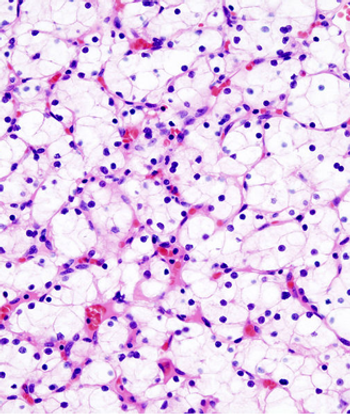
Scientists have discovered a new molecular target for therapy to fight clear cell renal cell carcinoma, neuronal pentraxin 2 (NPTX2).

The first results from a phase I trial of gene therapy for exudative age-related macular degeneration using a subretinal injection suggest that the treatment is safe and well tolerated even in the elderly population and may eliminate the need for frequent reinjection with anti-vascular endothelial growth factor agents.

Much work is being done with Adeno-associated virus (AAV) vectors for application in retinal gene therapy, with modifications being made to the capsid and genome of the vector to generate novel variants with unique transduction profiles, according to Shannon Boye, PhD.

Lymphocyte infusions are extremely effective therapy in patients with chronic myeloid leukemia who relapse after allogeneic hematopoietic stem cell transplantation, and the timing of the infusion is relatively unimportant, according to a new study.
As gatekeepers of the cell cycle, the cyclin-dependent kinases are often implicated in the progression of cancer and make prime targets for therapy.

Researchers safely infuse engineered immune cells in groundbreaking gene therapy study.