Hematology
Latest News
Latest Videos

Podcasts
CME Content
More News

Deevyashali Parekh, MBBS, an internal medicine resident at SUNY Upstate Medical University Hospital, discussed findings from a patient population traditionally excluded from clinical trials for CAR-T.

The chairman of the Division of Pediatric Hematology & Oncology and BMT at Cleveland Clinic Children's discussed results from the phase 1/2 RUBY trial he presented at ASH 2025.

ImmunoLogic cohost Janna Minehart, MD, discussed a few exciting immunotherapy sessions she attended at ASH's Annual Meeting.

The internal medicine resident at SUNY Upstate Medical University Hospital discussed findings from a patient population traditionally excluded from clinical trials for CAR-T.

Crawford Strunk, MD, an associate staff member at the Cleveland Clinic, discussed the institution's experience with integrating use of exa-cel and lovo-cel.

The clinical fellow in hematology/oncology at the University of Pennsylvania discussed several sessions she's excited about at the ASH Annual Meeting.

The associate staff member at the Cleveland Clinic discussed the institution's experience with integrating use of exa-cel and lovo-cel.

Crawford Strunk, MD, an associate staff member at the Cleveland Clinic, discussed a study he presented at ASH’s 2025 Annual Meeting.

The associate staff member at the Cleveland Clinic discussed a study he presented at ASH’s 2025 Annual Meeting.

AZD0120, a dual-targeted CAR T-cell therapy, shows high efficacy in treating relapsed multiple myeloma, achieving a 96% response rate.

Review top news and interview highlights from the week ending December 5, 2025.

The agency's decision was based on results from an r/r MZL cohort in the phase 2 TRANSCEND FL clinical trial.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

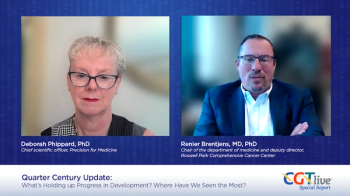
Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed lessons that should guide the next generation of developers and regulators entering the field.

In episode 7 of ImmunoLogic, Anusha Kalbasi, MD, discussed his research on the use of IL-9 to enhance CAR-T efficacy.

Review top news and interview highlights from the week ending November 28, 2025.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, spoke about streamlining collaboration to speed up advancement of new therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending November 21, 2025.

Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, spoke about strategies for streamlining development of novel therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The chair of the Division of Hematology at Mayo Clinic discussed practical considerations for the introduction of cell therapies for the treatment of hematologic malignancies.

Review top news and interview highlights from the week ending November 7, 2025.

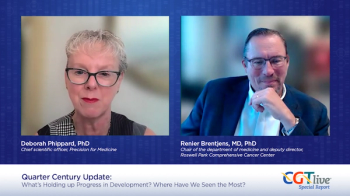
Deborah Phippard, PhD, and Renier Brentjens, MD, PhD, discussed major events in the past quarter century of cell and gene therapy research.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.