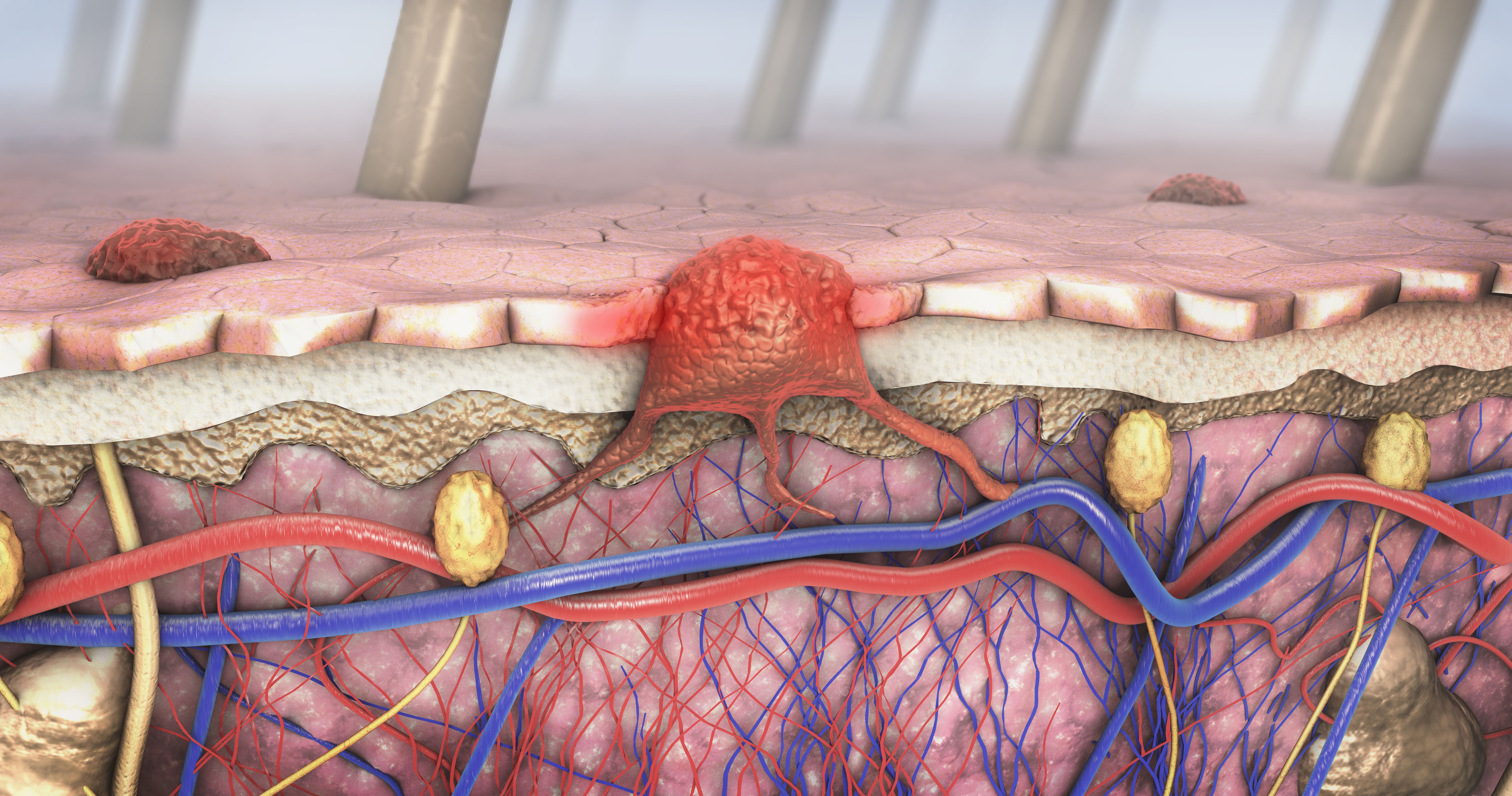
Oncology
Latest News
Latest Videos
CME Content
More News

Sarnaik discussed the investigational TIL therapy’s potential as an additional option for patients.
CAN-2409 transduces tumor cells with the thymidine kinase gene, sensitizes these cells to valacyclovir, and stimulates patients’ immune response.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

ExCellThera is in the process of the expanding the studies into new trial sites in the United States and Europe.

Updated data from a first-in-human trial were presented at the SITC 2022 meeting.

AFNT-111 showed anti-tumor activity in NSG mouse models of breast cancer, pancreatic cancer, and colon cancer.

The chief of research and immunotherapy at Cedars-Sinai The Angeles Clinic and Research Institute discussed updated data from the phase 2 C-144-01 study of the lifileucel TIL therapy.

The Genomics and Epigenetic Guided Safe Harbor mapper will aid in the future design of gene-editing therapies.

A2B530 and A2B694 target CEA and MSLN cells that have HLA loss of heterozygosity.
Among the 16 patients treated in the trial, 5 showed stable disease after treatment.

Review top news and interview highlights from the week ending November 11, 2022.

Data from the COBALT-RCC study in renal cell carcinoma were presented at SITC 2022.
The data included an IRC-assessed ORR of 31.4%.

The patient’s ECOG performance status remained at 0 from screening through the rest of trial participation.

The CRIPSR-edited neoantigen-specific T cell therapy demonstrated safety and feasibility in the first-in-human PACT-0101 study presented at SITC 2022.

Further analysis will be performed to elucidate which characteristics correlate with optimal cell therapy behavior.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The director of translational research in myeloma at the Tisch Cancer Institute discussed the relevance of the new research as more CAR T therapies come to market.

The assistant professor of Hematologic Oncology and Blood Disorders at Atrium Health discussed integrating CAR T therapy into treatment paradigms.

The chair of gynecologic oncology at Moffitt Cancer Center gave an overview of a phase 1 trial being conducted at Moffitt Cancer Center.

Review top news and interview highlights from the week ending November 4, 2022.
Data from the first 2 patients were presented at the IWWM 2022 meeting.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The chair of gynecologic oncology at Moffitt Cancer Center discussed advantages of targeting follicle stimulating hormone receptor with cell therapy for ovarian cancer.

MT-101 is designed for quick delivery to patients with a vein-to-vein time of 8 days.