
The associate professor from the Harold C. Simmons Comprehensive Cancer Center of UT Southwestern Medical Center discussed the latest data from the KarMMA trial.

The associate professor from the Harold C. Simmons Comprehensive Cancer Center of UT Southwestern Medical Center discussed the latest data from the KarMMA trial.
The early-phase data readout follows news that the FDA recently approved its IND for its CAR-T candidate in multiple myeloma.
Experts discussed data from trials in lymphoma presented at ASCO 2021.

The chief executive and chief medical officers of Celyad Oncology discussed their platform and positive data presented at EHA 2021.
Presentations at ISTH 2021 revealed attributes most important to patients when considering gene therapies and where further research is needed.

The World Federation of Hemophilia’s registry will include long-term safety and efficacy data in people with hemophilia treated with gene therapies.
No concerning effects to liver health were observed in the phase 1/2a study.

Phase 3 studies are continuing in the enhanced Padua FIX variant AMT-061 for hemophilia B.

The professor of internal medicine in the Division of Oncology and Hematology at the University of Nebraska Medical Center discussed novel treatment options for patients with hematologic malignancies.

The professor of internal medicine in the Division of Oncology and Hematology at the University of Nebraska Medical Center discussed the benefits of CAR T-cell for patients with follicular lymphoma.
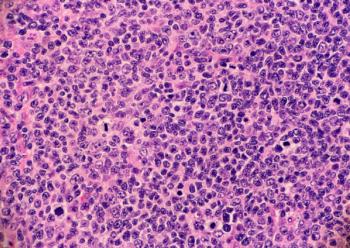
Stephen J. Schuster, MD, discussed tisagenlecleucel's efficacy and safety over other available treatments for relapsed/refractory FL.
A drop in endogenous Factor VIII expression was observed from treatment to 5-year follow-up despite continued demonstration of efficacy.
Manuel Litchman, MD, the president, chief executive officer, and director of Mustang Bio discussed the company’s lead and second program.

The president, chief executive officer, and director of Mustang Bio discussed the company’s pipeline.
Findings from an ongoing phase 1 trial were presented at the 2021 ASGCT meeting.

The founder and chief executive officer of SQZ Biotech discussed the potential of their APC platform to treat a variety of tumors.
Over 90% participants had an annualized bleeding rate of 0 or a lower bleed rate than baseline after week 4 after treatment.
Early safety and efficacy data support the use of natural killer T (NKT) cells in patients with stage IV relapsed/refractory neuroblastoma.

The director of the Mario Lemieux Center for Blood Cancers at UPMC Hillman Cancer Center discussed strategies to manage AEs associated with CAR T therapy.

The founder and chief executive officer of SQZ Biotech discussed the development and advantages of their proprietary Cell Squeeze technology in creating cell therapies.

The CEO of Avamab Pharma discussed how funding and research changes directions.

The director of Clinical Research in Hematologic Malignancies at Levine Cancer Institute discussed overall response rate and stringent complete responses in the CARTITUDE-1 trial.

The director of the Mario Lemieux Center for Blood Cancers at UPMC Hillman Cancer Center discussed how the meeting sets the foundation for what’s to come in the treatment of multiple myeloma and other hematologic malignancies.
The CEO of Avamab Pharma discussed how the field of gene therapy has shifted due to the COVID-19 pandemic.

The head of neuroscience at Sangamo Therapeutics discussed advances in understanding disease biology that allow for more specific gene targeting.

The head of neuroscience at Sangamo Therapeutics discussed the potential of ZFPs in central nervous system disorders.
The recommendation comes after positive data was released from the recent phase 2 KarMMa trial.

Interim data from a phase 1 study from Intellia Therapeutics and Regeneron Pharmaceuticals is the first to support in vivo CRISPR genome editing in humans.

Philippe Moreau, MD, discussed the CARTITUDE-1 trial's promising results with ciltacabtagene autoleucel read out at the 2021 ASCO Annual Meeting.

The director of Clinical Research in Hematologic Malignancies at Levine Cancer Institute discussed adverse events in the CARTITUDE-1 trial.