The National Comprehensive Cancer Network published a document intended for patients to understand how CAR-T cells work and what side effects are associated with the treatment.

The National Comprehensive Cancer Network published a document intended for patients to understand how CAR-T cells work and what side effects are associated with the treatment.

The European Medicines Agency has validated a Marketing Authorization Application for the CD19-directed CAR T-cell therapy lisocabtagene maraleucel for the treatment of adult patients with relapsed/refractory diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and grade 3B follicular lymphoma following at least 2 prior therapies.

Advances of chimeric antigen receptor T-cell therapy technologies are in rapid development and under investigation in a range of preclinical and clinical research around the globe.
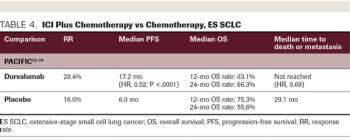
ABSTRACT Historically, platinum-based chemotherapy was the standard of care for metastatic lung cancer. However, since the success of immune checkpoint inhibitors (ICIs) in melanoma, PD-1/PD-L1 and CTLA-4 immune checkpoint pathways have been established as effective therapies to manage advanced non–small cell lung cancer (NSCLC) and extensive-stage (ES) small cell lung cancer (SCLC). Multiple large-scale randomized clinical trials have analyzed the effects of ICIs in NSCLC, and results of these trials have since translated to the approval of single-agent PD-1/PD-L1 inhibitors, and the combination of PD-1 inhibitors with platinum-based chemotherapy has become the new standard of care for patients with advanced NSCLC. Furthermore, in ES SCLC, in which chemotherapy or chemoradiation has been the standard of care for decades, 2 anti–PD-1/PD-L1 agents have been approved for use in the frontline setting for ES SCLC, in combination with chemotherapy. Despite progressive integration of immunotherapy into treatment regimens, there remains a need for reliable biomarkers to precisely determine therapy candidates.

Pralsetinib, an investigational precision therapy in late-stage development for individuals with alterations in the RET gene, would be jointly sold in the United States by the 2 companies if it is approved.
The FDA has issued a clinical hold on the phase 1 MELANI-01 trial evaluating the CAR T-cell product UCARTCS1A in the treatment of patient with relapsed/refractory multiple myeloma.

Lifleucel, a TIL therapy, is being investigated in patients with metastatic melanoma.

The patient guide explains 2 possible side effects, cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, that need to be caught quickly in order to be reversible.
Patients with chemotherapy-naïve, locally advanced, or metastatic non-small cell lung cancer who were treated with tiragolumab plus an anti-PD-L1 agent showed better efficacy versus single-agent checkpoint inhibitor therapy alone.

Immunotherapy and chimeric antigen receptor (CAR) T-cell therapy are new and important treatments revolutionizing care of some cancers; however, their side effects can be very different than what is seen in traditional approaches, such as chemotherapy.

There are promising signs that chimeric antigen receptor (CAR) T-cell therapies might lead to meaningful advances in the therapy of multiple myeloma. However, investigators will first need to clear a number of key hurdles.

Natalie Sophia Grover, MD, discusses ongoing research with CAR T-cell therapy in hematologic malignancies, efforts examining the potential for this approach in solid tumors, and future directions and challenges with this modality.
Patients with B-cell non-Hodgkin lymphomas and B-cell acute lymphoblastic leukemia can benefit greatly from chimeric antigen receptor (CAR) T-cell therapy, but providing that therapy has become much more difficult in the age of coronavirus disease 2019 (COVID-19).

Findings from an exploratory analysis of the phase 1/2 ZUMA-1 trial demonstrated clinical efficacy in patients with relapsed/refractory large B-cell lymphoma who were retreated with the CAR T-cell therapy axicabtagene ciloleucel.

Jesus G. Berdeja, MD, further discusses other exciting trials in multiple myeloma and CAR T therapy being presented at the 2020 ASCO Virtual Scientific Program.

The FDA granted accelerated approval to selinexor (Xpovio, Karyopharm Therapeutics) for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). The oral treatment is to be used after at least 2 lines of systemic therapy.

The FDA approved oral selinexor for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma after at least 2 lines of systemic therapy.

The FDA has approved selinexor for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.

The National Medical Products Administration in China has approved pembrolizumab for the treatment of patients with locally advanced or metastatic esophageal squamous cell carcinoma whose tumors express PD-L1 as determined by an approved test, following disease progression on 1 prior line of systemic therapy.

The connections between cancer and HIV/AIDS became clear relatively early in the HIV/AIDS pandemic and continue to this day. Not only were opportunistic infections present in a majority of HIV-infected patients who met the initial diagnostic criteria for AIDS, but several cancer types were far more prevalent as well. While there is still much to understand before HIV is fully conquered, we have already learned a great deal about the pathobiology of this virus that has helped advanced immune-oncological technologies and led to the development of increasingly effective gene therapy delivery systems.
Scientists at the Trinity College Dublin and University College London developed a new gene therapy approach that has the potential to treat a group of eye diseases known as retinitis pigmentosa (RP), according to research published in Stem Cell Reports.

Patients with relapsed chronic lymphocytic leukemia and B-cell lymphoma who received the anti-CD19 CAR T-cell therapy FMC63-28Z experienced highly durable rates of remission, according to long-term data from a phase 1/2 study.

Known as a gene therapy pioneer, Zaia has spent almost 40 years at City of Hope, in Duarte, California. He was first drawn by the promise of studying cytomegalovirus. Over the decades, his groundbreaking research has encompassed HIV/AIDS, cellular gene transfer therapy, immunotherapy, bispecific antibodies, and now hyperimmune globulin for workers on the frontlines of the coronavirus disease 2019 (COVID-19) pandemic.
Investigators reported in Lancet Oncology that second-line treatments are needed in small cell lung cancer, as few options exist for these patients once first-line therapy fails.

Selected abstracts from the American Diabetes Association's 80th Scientific Sessions discuss when to add injectable therapy, how patients who switched to semaglutide lost more weight and gained glycemic control, and offered results from an early-phase study on a monoclonal antibody that may preserve B-cell function.
Patients with relapsed/refractory T-cell lymphoblastic leukemia face poor outcomes, and are generally treated by salvage therapy followed by allogeneic hematopoietic stem cell transplant. A new study suggests an optimal option for salvage therapy.

Jesus G. Berdeja, MD, of the Sarah Cannon Research Institute discussed the CARTITUDE-1 study that examined CAR-T cell therapy to treat patients with relapsed/refractory multiple myeloma presented at the 2020 ASCO Virtual Scientific Program.

Treatment with the CAR T-cell product lisocabtagene maraleucel led to high response rates, with durable complete responses, in transplant-ineligible patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma who had poor prognostic features.

Data show the therapy’s positive safety and expression results from a small number of clinical trial participants with limb-girdle muscular dystrophy type 2E out to 1 year.

Omid Hamid, MD, highlights key abstracts in melanoma as they relate to the optimal duration and sequencing of checkpoint inhibitors, adoptive cell therapy, and subgroups of patients with mucosal melanoma and brain metastases.