
Despite an embrace of greater patient populations by the FDA, cardiovascular research into stem cell therapy has been slow and burdened.

Despite an embrace of greater patient populations by the FDA, cardiovascular research into stem cell therapy has been slow and burdened.

As safety and efficacy programs advance, clinicians consider the investigative therapy's potential in cardiology.

Novel strategies are needed to enhance the efficacy of CAR T-cell therapies in patients with acute lymphoblastic leukemia, including new constructs that target more than 1 antigen.

The FDA has granted a breakthrough therapy designation to the MET inhibitor tepotinib as a treatment for certain patients with metastatic non–small cell lung cancer with MET exon14-skipping alterations.

New data from clinical trials of ocrelizumab showed that the anti-CD20+ B cell therapy lowered serum NfL levels, and that the NfL levels offered prognostic value for disease progression in MS.
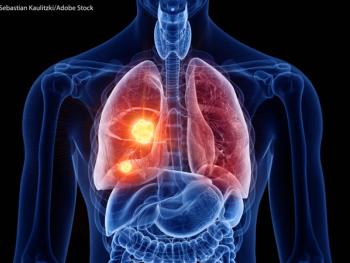
In this phase III trial, investigators assessed the clinical efficacy and safety of durvalumab with or without tremelimumab with etoposide and carboplatin or cisplatin chemotherapy followed by durvalumab with or without tremelimumab maintenance therapy compared with EP alone as first-line treatment in extensive-stage small-cell lung cancer.

Combination immunotherapy with nivolumab plus ipilimumab was examined as a first-line therapy for patients with advanced non–small-cell lung cancer. Results were presented at the International Associate for the Study of Lung Cancer 2019 World Conference on Lung Cancer.

The FDA has granted a fast track designation to AMG 510 for the treatment of patients with KRAS G12C–mutated metastatic non–small cell lung cancer who received prior therapy.

The FDA has granted a breakthrough therapy designation to capmatinib (INC280) as a first-line treatment for patients with MET exon14 skipping—mutated non–small cell lung cancer.

While the results are early, if further research proves the approach effective, it could help boost the impact of treatments like chimeric antigen receptor (CAR) T-cell therapy, which to date hasn’t had much luck in solid tumors.
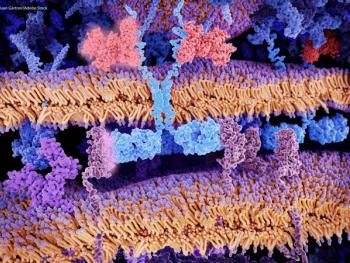
Delays in CAR T-cell therapy may significantly decrease gains in survival and productivity for patients with diffuse large B-cell lymphoma and pediatric acute lymphoblastic leukemia.

ONCOLOGY discussed therapy options, including chimeric antigen receptor (CAR)-T-cell therapies for pediatric acute lymphoblastic leukemia (ALL), with Susan R. Rheingold, MD, Medical Director of the Oncology Outpatient Clinic and attending physician with the Cancer Center at Children’s Hospital of Philadelphia.

The FDA has granted a priority review designation to a new drug application for zanubrutinib for the treatment of patients with mantle cell lymphoma who have received ≥1 prior therapy.

The emergence of monoclonal antibodies, immunomodulatory agents, immunotoxins, bispecific T-cell engagers, and CAR T-cell therapies will redefine multiple myeloma treatment. However, these new approaches, by themselves, are not enough to achieve cure; they must be used in combination.

Saul Priceman, PhD, City of Hope assistant professor in the Department of Hematology & Hematopoietic Cell Transplantation, and his research team have received a $9.28 million award from the California Institute for Regenerative Medicine to support a chimeric antigen receptor T cell phase 1 clinical trial for the treatment of women with HER2-positive breast cancer that has spread to the brain.

Treatment delays limit the social value generated by chimeric antigen receptor (CAR) T-cell therapy for the treatment of pediatric acute lymphoblastic leukemia and diffuse large B-cell lymphoma.

Following CMS’ decision to make chimeric antigen receptor (CAR) T-cell therapy available to Medicare beneficiaries nationwide, including in the community oncology setting, the Community Oncology Alliance (COA) applauded the decision.

Kieron Dunleavy, MD, discusses the current treatment paradigm in mantle call lymphoma and the emerging potential for CAR T-cell therapy.
In a long-awaited national coverage determination decision, CMS said Wednesday that it approved chimeric antigen receptor (CAR) T-cell therapies for Medicare beneficiaries nationwide.

Olaparib demonstrated a statistically significant and clinically meaningful improvement in radiographic progression-free survival compared with enzalutamide or abiraterone acetate in patients with metastatic castration-resistant prostate cancer who harbor a homologous recombination repair gene mutation and have progressed on prior therapy with either androgen receptor inhibitor, meeting the primary endpoint of the phase III PROfound trial.

Novartis hid manipulated data about its $2 million gene therapy Zolgensma from the FDA; US District Judge Kristine Baker granted a preliminary injunction preventing Arkansas from enforcing 3 abortion restrictions; a federal judge in Ohio expressed support for a plan by attorneys representing cities and counties suing US opioid manufacturers and distributors that would bring every US community into their settlement talks despite objections from most states.

The agency said the gene therapy should remain on the market while it assesses the situation and does not impact their evaluation of data from the human clinical trials.

CMS finalized additional payments for chimeric antigen receptor (CAR) T-cell therapies and adjusted Medicare payment policies for rural and urban hospitals for fiscal year 2020 by changing the inpatient hospital wage index.
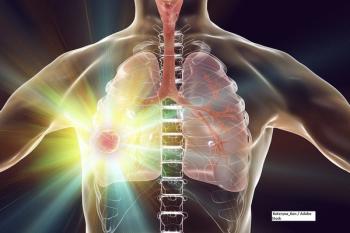
A phase III trial found bevacizumab and pemetrexed were effective as a maintenance therapy, but researchers do not recommend it to treat non–small-cell lung cancer.

Interim data from the first 8 pediatric patients showed that the AAV-CLN6 gene therapy demonstrated a positive impact on motor and language function compared to a natural history dataset, as well as in comparison to in-study sibling pairs.

In elderly patients with brain metastasis secondary to small cell lung cancer, whole brain radiation therapy was linked to a modest rise in survival.

The FDA has approved pembrolizumab for the treatment of patients with recurrent locally advanced or metastatic squamous cell carcinoma of the esophagus whose tumors express PD-L1 (combined positive score ≥10) as determined by an FDA-approved test, with disease progression after ≥1 prior lines of systemic therapy.
Orphan drug status applies to therapies that will treat fewer than 200,000 patients in the United States.

The investigational therapy has the potential to be a first-in-class neuronal progenitor cell therapeutic with anti-apoptotic activity that improves cerebral blood flow and neurological outcome in stroke.

Deepu Madduri, MD, discusses ongoing trials examining CAR T-cell therapy in myeloma and the potential to use it in earlier lines of therapy.