Research presented at AACR 2019 evaluated autologous mesothelin-targeted chimeric antigen receptor T-cell therapy in patients with malignant pleural disease.

Research presented at AACR 2019 evaluated autologous mesothelin-targeted chimeric antigen receptor T-cell therapy in patients with malignant pleural disease.
A phase I trial evaluated the safety and efficacy of adding lymphodepletion to HER2-targeted T-cell therapy in patients with advanced HER2-positive sarcoma.

The planned phase 1/2 trial of the recombinant AAV5 vector treatment, the first one-time administered AAV gene therapy to enter clinical testing for Huntington disease, is expected to begin dosing patients in the second half of 2019.

Researchers have identified a potential new target for CAR T-cell therapy in patients with multiple myeloma.

Tumor mutational burden identified patients who obtained a survival benefit with the PD-L1 inhibitor durvalumab, as initial therapy versus chemotherapy for advanced non–small cell lung cancer, even though the primary analysis of the randomized trial showed no difference between treatment groups, according to a new analysis.

Patients with multiple myeloma who relapsed following allogeneic stem cell transplantation and also failed several posttransplant therapies demonstrated a strong response to daratumumab salvage therapy.

CAR T cells targeting mesothelin-expressing tumors demonstrated safety and efficacy in a preliminary clinical evaluation in patients with malignant pleural disease.

The administration of HER2-directed CAR T-cell therapy and lymphodepletion chemotherapy demonstrated antitumor activity and was found to be safe in pediatric and adult patients with advanced HER2-positive sarcoma.

Although CAR T-cell therapy has not yet proved effective against solid tumors, a novel CAR that targets mesothelin proteins expressed in pancreatic cancer is showing early signs of activity.
Recent work looking at the response to immune checkpoint blockade (ICB) therapy given before surgery for advanced melanoma found increased infiltration of B cells, a type of white blood cell, in patients who responded to therapy compared with those who did not.

Sequential administration of CAR T-cell therapy targeting both the CD19 and CD22 antigens demonstrated high complete remission rates and improved long-term survival in patients with relapsed/refractory B-cell acute lymphoblastic leukemia following allogeneic hematopoietic stem cell transplant.

Lazaros J. Lekakis, MD, discusses the promise of CAR T cells and some unanswered questions with this rapidly emerging therapy.

Experts shared the challenges they've encountered in integrating novel treatments such as CAR T-cell therapy into practice at the 2019 NCCN Annual Conference.

A panel during the opening day of the 2019 National Comprehensive Cancer Network Annual Conference examined the recent process for National Coverage Determination for chimeric antigen receptor (CAR) T-cell therapy and what it means for the future of innovative treatments.

Napabucasin, a novel therapy that targets cancer stem cell pathways, is being investigated in a phase III clinical trial that is believed to be the largest study ever conducted in pancreatic ductal adenocarcinoma in the metastatic setting

Sergio A. Giralt, MD, discusses recent data with allogeneic hematopoietic stem cell therapy and why this long-standing modality remains an integral part of treatment for patients with relapsed myeloma.

The Community Oncology Alliance (COA) has submitted formal comments to CMS regarding the agency's proposed national coverage determination for chimeric antigen receptor (CAR) T-cell therapy.

There is concern among stakeholder groups that references to “hospital” could mean that community practices would be unable to be reimbursed by Medicare under a proposed National Coverage Determination.

Although these therapies were initially conceived of and developed as inpatient therapies, interest is growing in extending chimeric antigen receptor T-cell therapies to the outpatient setting.

In a rare clinical trial, stem cell transplantation appeared to provide greater protection against MS progression than DMT.

Noopur Raje, MD, discusses the current treatment landscape of multiple myeloma, with a specific focus on available triplet regimens and the recent data with chimeric antigen receptor T-cell therapy.
The PRECIS study looked at consolidation treatment with autologous stem cell transplantation vs whole-brain radiation therapy in younger patients with CNS lymphoma.
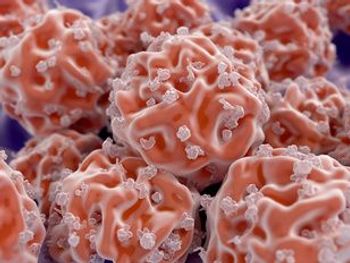
Two abstracts presented at the Transplantation and Cellular Therapy Meetings analyzed the detection of minimal residual disease during and after hematopoietic stem cell transplantation.

Real-world data on the use of chimeric antigen receptor T-cell therapy in large B-cell lymphoma bear out the pivotal results from ZUMA-1 and demonstrate not only that the treatment approach is here, but also that it’s time to address issues of efficacy, safety, cost, and moving this approach into earlier lines of therapy.

B-cell maturation antigen-specific chimeric antigen receptor T-cell therapy is delivering impressive results in multiple myeloma, demonstrating durable responses and acceptable toxicities.

Despite being labeled as rare diseases, a number of neurologic conditions impact more patients than most would believe. The consultant with expertise in ophthalmology, gene therapy, and rare and orphan diseases, chimed in about how these diseases can often be overlooked.

Chimeric antigen receptor T cells targeting CD30 proved to be safe and active in relapsed/refractory Hodgkin lymphoma.

Every week, The American Journal of Managed Care® recaps the top managed care news of the week, and you can now listen to it on our podcast, Managed Care Cast.

Frederick L. Locke, MD, discusses the optimal management of chimeric antigen receptor T-cell therapy-related adverse events.

Patients with relapsed/refractory chronic lymphocytic leukemia had an improved response rate with chimeric antigen receptor T-cell therapy if they also received ibrutinib.