The recommended phase 2 dose of cilta-cel elicited deep and durable responses, along with a tolerable safety profile.

The recommended phase 2 dose of cilta-cel elicited deep and durable responses, along with a tolerable safety profile.
Positive data has been seen with CAR T-cell therapy in patients with indolent lymphoma.
Experts discussed using newer targeted treatment approaches as frontline therapy instead of transplant in patients with relapsed/refractory diffuse large B-cell lymphoma.
A 1-stage factor VIII assay revealed no apparent decrease in factor VIII activity over time.

Review top news and interview highlights from the week ending November 19, 2021.
Experts discussed using CD19-targeted agents in patients with various subtypes of diffuse large B-cell lymphoma.

The hematologist at Ohio State University Comprehensive Cancer Center–The James discussed utilization rates of autologous stem cell transplant in patients with mantle cell lymphoma.

AJ Joshi, MD, chief medical officer, Atara Biotherapeutics, discussed data from the ALLELE study to be presented at ASH 2021.
A reduction in late mortality among patients who received allogeneic blood or marrow transplantation in the last 40 years was limited to those who received treatment at a younger age.

Dan Oliver, cofounder and chief executive officer, Rejuvenate Bio, discussed the company’s ultimate goal of reversing aging.
The NK cell therapy is also being evaluated in a phase 1 study for acute myeloid leukemia.

The professor of medicine at Duke University School of Medicine previously served as commissioner in 2016.

The hematologist from Ohio State University Comprehensive Cancer Center–The James discussed the impact of socioeconomic factors on completion of autologous stem cell transplant in patients with mantle cell lymphoma.
Overall survival was 72% at 12 months but dropped to 54% at 14.6 months.
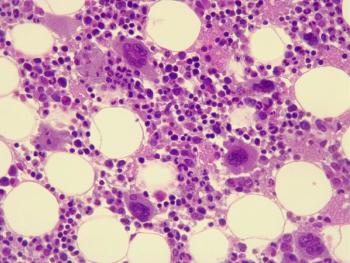
The request aims to ensure the comparability of omidubicel manufactured at different sites.
The director of Lymphoma Clinical Research at the University of Texas MD Anderson Cancer Center, discussed the future of DLBCL treatment.

Review top news and interview highlights from the week ending November 12, 2021.
The first in-human study of CAR macrophages has dosed 2 participants so far.

The associate professor of medicine from MD Anderson Cancer Center discussed data from the phase 1 cohort of liso-cel combined with ibrutinib.
Three patients dosed had tumors shrinkages of 18%, 21%, and 27%.
The hematologist from Moffitt Cancer Center discussed the FDA approval of brexucabtagene autoleucel in relapsed/refractory B-cell acute lymphoblastic leukemia.
Data from the IMA203 trial were presented at the SITC 2021 annual meeting.
Sonny Hsiao, PhD, chief executive officer, president and cofounder, Acepodia, discussed the company’s future research and plans.

The director of Myeloma Immunotherapy at University of Pennsylvania discussed ciltacabtagene autoleucel's safety profile.
TCR² Therapeutics is collaborating with Bristol Myers Squibb to evaluate combination therapies in a phase 2 trial.