
The chief of Cancer Immunotherapy at Rutgers Cancer Institute of New Jersey discussed unmet needs with cellular therapies in metastatic epithelial cancer.
AUTO1 CAR T Therapy Improves EFS in Relapsed/Refractory Acute Lymphoblastic Leukemia

The chief of Cancer Immunotherapy at Rutgers Cancer Institute of New Jersey discussed unmet needs with cellular therapies in metastatic epithelial cancer.

The hematologists from Moffitt Cancer Center and MD Anderson Cancer Center discussed the need for collaboration between academic and community practices for patients who receive CAR T-cell therapy.
Christopher R. Flowers, MD, reviewed challenges of treating indolent B-cell lymphoma.
Patients in the liso-cel arm had more favorable overall least square mean changes from baseline to day 126 vs the SOC arm in quality of life scores.

CYNK-001, a non-genetically modified cryopreserved human placental hematopoietic stem cell-derived natural killer cell therapy, is in development for the potential treatment of patients with acute myeloid leukemia.
Review some of our most-viewed coverage of advancements in cell therapies, including the latest study results and insights from experts in the field.

The associate professor from Perelman School of Medicine, University of Pennsylvania, discussed co-administered CART22-65s and huCART19 in relapsed/refractory acute lymphoblastic leukemia.

Review top news and interview highlights from the week ending December 24, 2021.
Patients treated with liso-cel had a probability of continued response at 2-years of 49.5%.

The director of the hemostasis and thrombosis program at Children’s Hospital Los Angeles discussed the ATLAS-INH study of fitusiran.
Poseida’s announcement follows their presentation of positive data in metastatic castrate-resistant prostate cancer in September 2021.
The program is still on clinical hold following SUSARs of myelodysplastic syndrome in treated participants.
The FDA has placed a partial clinical hold on the program for patients that are under the age of 18 after an adolescent patient developed persistent anemia.

The professor from The University of Texas MD Anderson Cancer Center discussed long-term follow-up analysis of the phase 2 ZUMA-5 trial.
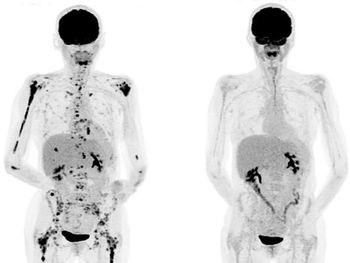
PET/CT plays a central role in evaluating response after CAR T-cell therapy.

Review top news and interview highlights from the week ending December 17, 2021.

Safety and the recommended dose of CYAD-211 and the lymphodepletion regimen served as the primary end point of the study.
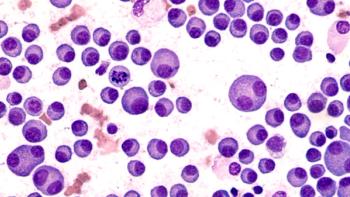
Patients treated with cilta-cel had a 76% reduction in the risk of death compared to patients treated with physician's choice of treatment.

The vice chair, Blood and Marrow Transplant and Cellular Immunotherapy Program, and co-leader, Immuno-Oncology, Moffitt Cancer Center, discussed the results of the phase 3 ZUMA-7 trial.

AJ Joshi, MD, chief medical officer, Atara Biotherapeutics, discussed safety findings from the phase 3 ALLELE study.
Annualized bleeding rate, spontaneous bleeding, and joint bleeding rates were all reduced in patients dosed with fitusiran compared to those receiving on-demand treatment.
Over half of patients in the fitusiran arm of the ATLAS-INH study had 0 treated bleeding events.
The hematologist from Moffitt Cancer Center and MD Anderson Cancer Center discussed CAR T-cell therapy and other treatment options for MCL.
The findings, which were simultaneously reported in the New England Journal of Medicine, support beti-cel as a potentially curative, one-time treatment option for these patients.
ADI-001 does not require genetic engineering to remove TCRs to avoid GvHD, making them ideal for an off-the-shelf cell therapy.