
Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

A third price model to be tested will limit the price of generic drugs for chronic conditions to $2 under Medicare plan D.

An independent DSMB has also recommended continued enrollment in the trial’s first cohort.

The professor and director of the Sen. Paul D. Wellstone Muscular Dystrophy Specialized Research Center at University of Washington School of Medicine discussed the paper he recently coauthored.

Review top news and interview highlights from the week ending February 10, 2023.

Mehra and Subramanian discussed preclinical safety studies with the gene therapy SLS-004 and plans for future research.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending February 3, 2023.

Mehra and Subramanian discussed preclinical research with the investigational gene therapy SLS-004.

Cartesian Therapeutics’ Descartes-08 will be administered as 6 weekly infusions and does not require preconditioning chemotherapy.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
The dosing of additional patients in a double-blind, placebo-controlled design will help support a BLA submission for TSHA-120.
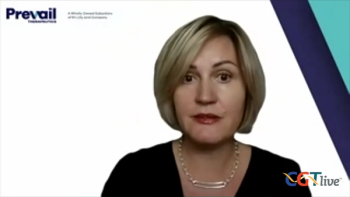
Olga Uspenskaya, MD, PhD, vice president, clinical development, Prevail Therapeutics, discussed the PROCEED trial of PR001.

Review top news and interview highlights from the week ending January 27, 2023.
No dose-limiting toxicities were observed at 28 days of follow-up.
The DGX-A01 study met its primary safety and efficacy endpoints in its highest dose cohort of IDCT.

Patients continued to show statistically significant upper limb performance improvements.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

With confidence building, numerous cell and gene therapies will likely go before the FDA and other global regulatory agencies this year, in addition to key data readouts.

REGENXBIO has also initiated recruitment for AFFINITY BEYOND, an observational study assessing the prevalence of AAV8 antibodies in male patients with DMD.

Review top news and interview highlights from the week ending January 20, 2023.

The FDA has lifted a clinical hold placed in the summer of 2022 due to a mild but medically significant case of peripheral sensory neuropathy.

The company is reprioritizing to focus on its clinical stage programs, which ran into a number of setbacks in 2022.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.