Neurology
Latest News

Latest Videos

CME Content
More News

Taysha also provided updates on its gene therapy program for Rett syndrome.

Benitec Biopharma is planning to initiate a phase 1b/2a clinical trial to evaluate BB-301.

Detailed topline data will be presented at the 2023 International Congress on Parkinson’s Disease and Movement Disorders in August.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending June 23, 2023.

Delandistrogene moxaparvovec treatment will reportedly cost $3.2 million.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

uniQure deemed the safety profile for AMT-130 to be manageable.

Data from a phase 1/2 clinical trial of NRTX-1001 were presented at the ISSCR 2023 Annual Meeting.

The advocacy groups are funding a 1-year study to be led by Barry Byrne, MD, PhD, at University of Florida.

Review top news and interview highlights from the week ending June 16, 2023.
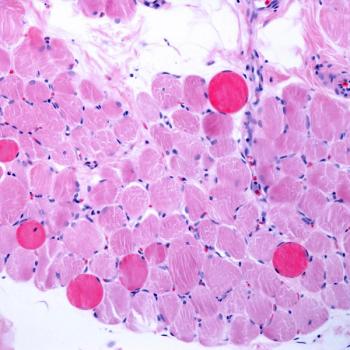
CGTLive takes a look at challenges that have beset the first gene therapy coming close to market for treating Duchenne muscular dystrophy in 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending June 9, 2023.

For International Batten Awareness Day, held annually on June 9, CGTLive reached out to several experts to inquire about the potential impact of gene therapy on the Batten disease treatment landscape.

The FDA’s Advisory Committee meeting is scheduled for September 27, 2023, and the PDUFA date for the company’s BLA review is set for December 8, 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The REVEAL trial is being carried out in Canada under a CTA that was cleared by Health Canada in March 2022.

Review top news and interview highlights from the week ending June 2, 2023.

In more positive news, the company’s phase 2 trial for treating DMD will enroll patients in the second half of 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The chief scientific officer of LocanaBio discussed preclinical data with an AAV-delivered, snRN, exon 51 skipping approach presented at ASGCT 2023.

Florian Eichler, MD, a neurologist at Massachusetts General Hospital, discussed efficacy data from the CANaspire clinical trial that he presented at ASGCT 2023 showing reductions in urine NAA levels.

There were no dose-limiting toxicities, serious adverse events, nor infusion-related reactions among 6 patients treated with ENCell’s investigational EN001 therapy.

Review top news and interview highlights from the week ending May 26, 2023.