Oncology
Latest News
Latest Videos

CME Content
More News
The FDA has pushed cilta-cel's BLA PDUFA date back by almost 4 months.
The vaccination therapy improved progression-free survival by 3.1 months over standard care in 1 patient treated.

The hematologist/oncologist from UCSF Helen Diller Family Comprehensive Cancer Center discussed the potential of vaccine-based therapies in multiple myeloma.

André Choulika, PhD, chief executive officer and cofounder, Cellectis, discussed the company’s expansion into gene therapies.
Ide-cel's March 2021 FDA approval marks a significant advance in relapsed/refractory multiple myeloma treatment.
Rick Fair, president and chief executive officer, Bellicum Pharmaceuticals, discussed the company’s programs and future plans.
Doctors debated the roles of allogeneic hematopoietic stem cell transplant and CAR T-cell therapy for the treatment of aggressive B-cell lymphoma.

The assistant professor of medicine at the Medical College of Wisconsin discussed immune-compromising factors that are indigenous to CAR T-cell therapy recipients.

Review top news and interview highlights from the week ending October 29, 2021.
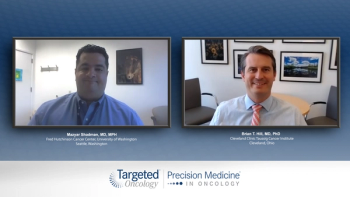
Targeting CD19 With CAR T in R/R DL B-Cell Lymphoma: Brian T. Hill, MD, PhD; Mazyar Shadman, MD, MPH
Doctors from University of Washington and Cleveland Clinic discussed targeting CD19 in CAR T-cell therapy for relapsed/refractory DLBCL.
Liso-cel is at the forefront of clinical development for patients with chronic lymphocytic leukemia.

Jessica Baker Flechtner, PhD, chief scientific officer, Genocea, discussed the company’s focus on solid tumors and manufacturing cell therapies.

Josh Ludwig, global director, commercial operations, ScaleReady, discussed making cell and gene therapy widely practical and viable for patients with cancer.

André Choulika, PhD, chief executive officer and cofounder, Cellectis, discussed the company’s partnerships and science.

bluebird bio is also planning to withdraw the marketing authorization for their β-thalassemia therapy, beti-cel, from the EU and UK.
Michael Wang, MD, discussed the evolution of treatment in the MCL space specifically with the emergence of chimeric antigen receptor T cells.

The hematologist from Moffitt Cancer Center discussed the promise of ALLO-715 in relapsed/refractory multiple myeloma.

The hematologist and medical oncologist discussed next steps with CAR T-cell therapy in penta-refractory multiple myeloma.

Jake Becraft, PhD, chief executive officer and cofounder, Strand Therapeutics, discussed the company’s future research and plans.

Review top news and interview highlights from the week ending October 22, 2021.

Rick Fair, president and chief executive officer, Bellicum Pharmaceuticals, discussed the company’s GoCAR, iMC, and caspaCIDe technologies.
The phase 1 study met its primary endpoints, with 97% of the CAR T-cell therapies reaching the protocol-specified dose and no dose-limiting toxicities during dose escalation.
Experts discussed challenges with treating relapsed or refractory diffuse large B-cell lymphoma.

The assistant professor of medicine from Medical College of Wisconsin discussed the potential impact of CAR T-cell therapy on the efficacy of COVID-19 vaccinations.

The professor of medicine at Washington University School of Medicine in St. Louis discussed the potential utility of frontline CAR T-cell therapy in mantle cell lymphoma.