Oncology
Latest News
Latest Videos

CME Content
More News

The hematologist/oncologist from Mayo Clinic discussed the growing presence of CAR T-cell therapies in lung cancer and melanoma.
Findings from the phase 2 ENSIGN and ELIANA studies suggest DNA sequencing predicts ALL relapse.
Event-free survival was 68.3% at 6 months, 48.3% at 12 months, and 48.3% at 24 months in patients treated with AUTO1.

The hematologist/oncologist from Rutgers Cancer Institute of New Jersey discussed ongoing research examining the CAR T-cell therapy ciltacabtagene autoleucel.

The chief of Cancer Immunotherapy at Rutgers Cancer Institute of New Jersey discussed unmet needs with cellular therapies in metastatic epithelial cancer.

The hematologists from Moffitt Cancer Center and MD Anderson Cancer Center discussed the need for collaboration between academic and community practices for patients who receive CAR T-cell therapy.
Christopher R. Flowers, MD, reviewed challenges of treating indolent B-cell lymphoma.
Patients in the liso-cel arm had more favorable overall least square mean changes from baseline to day 126 vs the SOC arm in quality of life scores.

CYNK-001, a non-genetically modified cryopreserved human placental hematopoietic stem cell-derived natural killer cell therapy, is in development for the potential treatment of patients with acute myeloid leukemia.

Review some of our most-viewed coverage of advancements in cell therapies, including the latest study results and insights from experts in the field.

The associate professor from Perelman School of Medicine, University of Pennsylvania, discussed co-administered CART22-65s and huCART19 in relapsed/refractory acute lymphoblastic leukemia.

Review top news and interview highlights from the week ending December 24, 2021.
All patients achieved a complete response at 3 months.

Patients treated with liso-cel had a probability of continued response at 2-years of 49.5%.

The director of the hemostasis and thrombosis program at Children’s Hospital Los Angeles discussed the ATLAS-INH study of fitusiran.
Poseida’s announcement follows their presentation of positive data in metastatic castrate-resistant prostate cancer in September 2021.

The professor from The University of Texas MD Anderson Cancer Center discussed long-term follow-up analysis of the phase 2 ZUMA-5 trial.
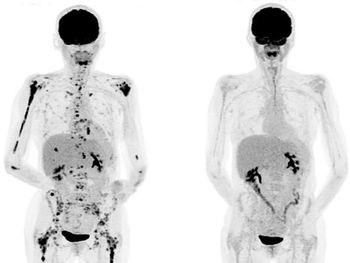
PET/CT plays a central role in evaluating response after CAR T-cell therapy.

Review top news and interview highlights from the week ending December 17, 2021.

Safety and the recommended dose of CYAD-211 and the lymphodepletion regimen served as the primary end point of the study.
Patients treated with cilta-cel had a 76% reduction in the risk of death compared to patients treated with physician's choice of treatment.

The vice chair, Blood and Marrow Transplant and Cellular Immunotherapy Program, and co-leader, Immuno-Oncology, Moffitt Cancer Center, discussed the results of the phase 3 ZUMA-7 trial.

AJ Joshi, MD, chief medical officer, Atara Biotherapeutics, discussed safety findings from the phase 3 ALLELE study.
The hematologist from Moffitt Cancer Center and MD Anderson Cancer Center discussed CAR T-cell therapy and other treatment options for MCL.
ADI-001 does not require genetic engineering to remove TCRs to avoid GvHD, making them ideal for an off-the-shelf cell therapy.