
Serious adverse events of reduced visual acuity and eye inflammation were observed in 2 clinical trials.

Serious adverse events of reduced visual acuity and eye inflammation were observed in 2 clinical trials.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

NR082 is being evaluated in a phase 1/2/3 clinical trial in patients with Leber hereditary optical neuropathy.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

A third price model to be tested will limit the price of generic drugs for chronic conditions to $2 under Medicare plan D.
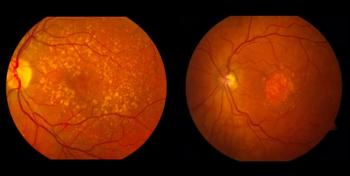
No serious adverse events deemed related to RGX-314 occurred in the treated patients.

Encouraging efficacy signals have also been observed in treated participants.

Review top news and interview highlights from the week ending February 10, 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The new findings are consistent with previous research, in which ophNdi1 demonstrated benefit in AMD models.

The company is developing a one-time, non-AAV2 gene replacement therapy to restore, treat, and prevent blindness of patients with RPE65 mutation-associated retinopathies.

Review top news and interview highlights from the week ending February 3, 2023.

The clinical hold comes a few weeks after the company announced it was stopping enrollment in the phase 1/2 clinical trial.

The phase 1 clinical trial is the second of Frontera’s trials to begin dosing in 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

MCO-010 is in phase 2 clinical trials for treating retinitis pigmentosa and Stargardt disease.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

With confidence building, numerous cell and gene therapies will likely go before the FDA and other global regulatory agencies this year, in addition to key data readouts.
Investigators analyzed data from 3 phase 3 studies and a long-term follow-up study.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

It was previously announced that CT103A received FDA clearance of its investigational new drug application for the treatment of relapsed/refractory (r/r) multiple myeloma.

A phase 1 clinical trial of EXG102-031 is expected to initiate in the first quarter of 2023.

The director of clinical research at Sierra Eye Associates discussed data from the phase 1/2 PRISM study.