
This is the first gene therapy approved in the US targeting mutations in a specific gene.

This is the first gene therapy approved in the US targeting mutations in a specific gene.
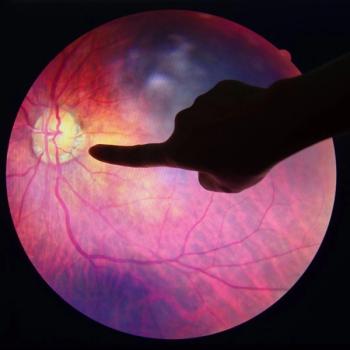
The retinal injection therapy could be capable of replacing damaged cells integral to eye vision.

Luxturna, an injection therapy, helped genetic vision-loss patients navigate mazes in a phase 3 trial.
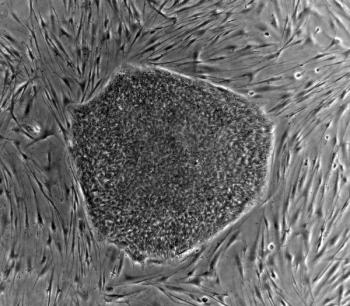
Companies are skirting regulation to be the first to build the next big stem cell therapy, creating clinical concerns and poor patient outcomes.

Jeffrey Heier talks about RGX-314 gene therapy and the advantages of subretinal delivery.

If approved, LUXTURNA would also be the first pharmacological treatment for inherited retinal disease

A new gene therapy tested the efficacy of injecting a virus into the eye.

Treatment with recombinant adeno-associated virus vector gene-therapy was shown to be safe and potentially effective in a small test group of patients with neovascular age-related macular degeneration.

According to Henry Kaplan, MD, University of Louisville School of Medicine, "One has to recognize that there are multiple approaches like gene therapy, neuroprotection, stem cell transplantation, and pharmacologic manipulation of other genes really holds the greatest benefit in terms of trying to reverse the inevitable loss of vision."
Gene therapy with the CRISPR/Cas9 system corrects mutations identified with patient-specific models that use stem cells generated from skin.

Study results show that transplanted human retinal stem cells rescue photoreceptors in rabbit retinas for 8 months.