
The study found significant improvements in BCVA, in contrast to several other studies evaluating Luxturna.

The study found significant improvements in BCVA, in contrast to several other studies evaluating Luxturna.

The data, presented at ARVO's 2023 conference, also showed a favorable safety profile.

Review top news and interview highlights from the week ending April 28, 2023.
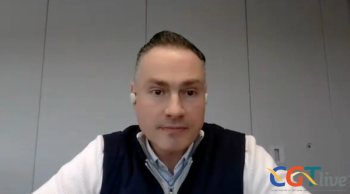
The chief scientific officer of Aurion Biotech discussed the properties of the cell therapy for corneal dystrophies, which was recently approved in Japan.


The new data builds upon positive long-term follow-up data from clinical trials.

The head of the Department of Ophthalmology at the Center for Medical Genetics, Ghent University Hospital, discussed the results of a post-marketing study presented at ARVO’s 2023 conference.

The safety profile of lenadogene nolparvovec at 5-years post treatment was similar to 2-year data.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

No serious TEAEs have been related to ATSN-101 with data up to 6 months after treatment.
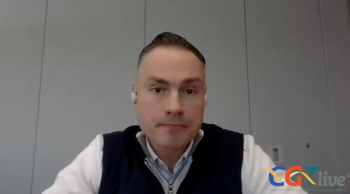
The chief scientific officer of Aurion Biotech discussed the investigational cell therapy AURN001.

STZ eyetrial’s treatment rAAV8.hPDE6A was otherwise deemed well-tolerated.

The commercially available gene therapy, developed by Spark Therapeutics, was evaluated in multiple studies presented at ARVO’s 2023 conference.
The new positive data on the AAV based gene therapy builds upon real-world data presented at ARVO’s conference in 2022.

The investigators from Children’s Hospital of Philadelphia discussed the follow-up studies they are conducting in light of their recent findings regarding DNA virus replication.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
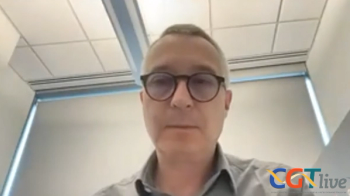
The investigators from Children’s Hospital of Philadelphia discussed the potential implications of their research into human adenoviruses for the development of new gene delivery tools.

All treated eyes had stable or improved MLMT scores at 6 or 9 months of follow-up.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Study authors Matthew Charman, PhD, and Matthew D. Weitzman, PhD, elaborated on the findings and implications of their research.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Atsena Therapeutics’ AAV gene therapy is being evaluated in 15 patients with Leber congenital amaurosis caused by biallelic mutations in GUCY2D across 5 cohorts of various doses. Additional data are expected to be presented later this year.

The clinical trial of HG004 for RPE65-LCA2 dosed its first patient in February 2023.

Review top news and interview highlights from the week ending March 31, 2023.

Most patients with RP treated with MCO-010 had clinically meaningful improvements in vision.