
Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

The associate professor of dermatology at Stanford University discussed the potential impact of Vyjuvek’s approval in the derm field.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

These, among many others, are some of the key clinical trials of gene, cell, and regenerative therapies that the CGTLive staff will be following throughout 2024.

Take a look at the stories that stood out as pillars of progress and success in endocrinology gene and cell therapy development in 2023.

This year we reached out to numerous experts outside the context of the conference cycle to seek their insight on company announcements, pipeline therapies, and disease awareness days.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Take a look at the stories that stood out as pillars of progress and success in dermatology gene and cell therapy development in 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Throughout 2023, interest in the application of CAR-T and other cell therapies to B-cell-driven autoimmune disease skyrocketed, with a multitude of clinical trials being initiated by various companies.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending December 8, 2023.

The associate professor of dermatology at Stanford University discussed his experiences investigating and helping to develop Vyjuvek, approved for treating DEB.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.

Review top news and interview highlights from the week ending December 1, 2023.

Catch up on the latest news, breakthroughs, and announcements from biotechnology companies making advancements in cell and gene therapies.
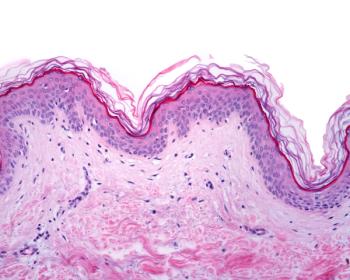
Pz-cel has a PDUFA date of May 25, 2024.
CHMP is expected to give an opinion on the MAA in the second half of next year.

CGTLive spoke to experts in the field about their experiences and impressions of B-VEC almost half a year after its approval for dystrophic epidermolysis bullosa.

M. Peter Marinkovich, MD, and Alfonso L. Sabater, MD, PhD, offered their respective perspectives on the recent FDA approval of the topical and redosable gene therapy for DEB.

The Krystal Biotech treatment, marketed as Vyjuveck, is now the first topical gene therapy for patients with DEB—marking a milestone in the paradigm of care.